Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with β-thalassemia major
- PMID: 22271905
- PMCID: PMC3366648
- DOI: 10.3324/haematol.2011.049957
Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with β-thalassemia major
Abstract
Background: Prospective data on cardiac iron removal are limited beyond one year and longer-term studies are, therefore, important.
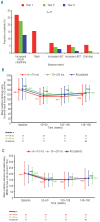
Design and methods: Seventy-one patients in the EPIC cardiac substudy elected to continue into the 3(rd) year, allowing cardiac iron removal to be analyzed over three years.
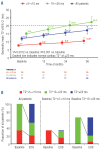
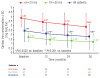
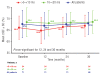
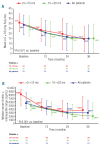
Results: Mean deferasirox dose during year 3 was 33.6 ± 9.8 mg/kg per day. Myocardial T2*, assessed by cardiovascular magnetic resonance, significantly increased from 12.0 ms ± 39.1% at baseline to 17.1 ms ± 62.0% at end of study (P<0.001), corresponding to a decrease in cardiac iron concentration (based on ad hoc analysis of T2*) from 2.43 ± 1.2 mg Fe/g dry weight (dw) at baseline to 1.80 ± 1.4 mg Fe/g dw at end of study (P<0.001). After three years, 68.1% of patients with baseline T2* 10 to <20 ms normalized (≥ 20 ms) and 50.0% of patients with baseline T2* >5 to <10 ms improved to 10 to <20 ms. There was no significant variation in left ventricular ejection fraction over the three years. No deaths occurred and the most common investigator-assessed drug-related adverse event in year 3 was increased serum creatinine (n = 9, 12.7%).
Conclusions: Three years of deferasirox treatment along with a clinically manageable safety profile significantly reduced cardiac iron overload versus baseline and normalized T2* in 68.1% (32 of 47) of patients with T2* 10 to <20 ms.
Figures
Similar articles
-
Continued improvement in myocardial T2* over two years of deferasirox therapy in β-thalassemia major patients with cardiac iron overload.Haematologica. 2011 Jan;96(1):48-54. doi: 10.3324/haematol.2010.031468. Epub 2010 Nov 11. Haematologica. 2011. PMID: 21071497 Free PMC article.
-
Efficacy of deferasirox in reducing and preventing cardiac iron overload in beta-thalassemia.Blood. 2010 Mar 25;115(12):2364-71. doi: 10.1182/blood-2009-04-217455. Epub 2009 Dec 8. Blood. 2010. PMID: 19996412 Clinical Trial.
-
Efficacy and safety of deferasirox at low and high iron burdens: results from the EPIC magnetic resonance imaging substudy.Ann Hematol. 2013 Jan;92(2):211-9. doi: 10.1007/s00277-012-1588-x. Epub 2012 Oct 21. Ann Hematol. 2013. PMID: 23086508 Free PMC article.
-
Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions.Blood. 2006 May 1;107(9):3436-41. doi: 10.1182/blood-2006-02-002394. Blood. 2006. PMID: 16627763 Free PMC article. Review.
-
Deferasirox: a review of its use for chronic iron overload in patients with non-transfusion-dependent thalassaemia.Drugs. 2014 Jun;74(9):1017-27. doi: 10.1007/s40265-014-0238-0. Drugs. 2014. PMID: 24919862 Review.
Cited by
-
Characterisation of individual ferritin response in patients receiving chelation therapy.Br J Clin Pharmacol. 2022 Aug;88(8):3683-3694. doi: 10.1111/bcp.15290. Epub 2022 Mar 26. Br J Clin Pharmacol. 2022. PMID: 35199367 Free PMC article.
-
Risk factors for endocrine complications in transfusion-dependent thalassemia patients on chelation therapy with deferasirox: a risk assessment study from a multi-center nation-wide cohort.Haematologica. 2022 Feb 1;107(2):467-477. doi: 10.3324/haematol.2020.272419. Haematologica. 2022. PMID: 33406815 Free PMC article.
-
Longitudinal monitoring of cardiac siderosis using cardiovascular magnetic resonance T2* in patients with thalassemia major on various chelation regimens: a 6-year study.Am J Hematol. 2013 Aug;88(8):652-6. doi: 10.1002/ajh.23469. Epub 2013 Jun 28. Am J Hematol. 2013. PMID: 23640778 Free PMC article. Clinical Trial.
-
Pancreatic T2* Magnetic Resonance Imaging for Prediction of Cardiac Arrhythmias in Transfusion-Dependent Thalassemia.J Clin Med. 2023 Sep 16;12(18):6015. doi: 10.3390/jcm12186015. J Clin Med. 2023. PMID: 37762955 Free PMC article.
-
Deferasirox nephrotoxicity-the knowns and unknowns.Nat Rev Nephrol. 2014 Oct;10(10):574-86. doi: 10.1038/nrneph.2014.121. Epub 2014 Jul 22. Nat Rev Nephrol. 2014. PMID: 25048549 Review.
References
-
- Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2(8653):27–30. - PubMed
-
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93. - PubMed
-
- Pepe A, Meloni A, Capra M, Cianciulli P, Prossomariti L, Malaventura C, et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative Magnetic Resonance Imaging. Haematologica. 2011;96(1):41–7. - PMC - PubMed
-
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360(9332):516–20. - PubMed
-
- Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

