Protective role of D-amino acid oxidase against Staphylococcus aureus infection
- PMID: 22271930
- PMCID: PMC3318415
- DOI: 10.1128/IAI.06214-11
Protective role of D-amino acid oxidase against Staphylococcus aureus infection
Abstract
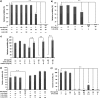
D-Amino acid oxidase (DAO) is a hydrogen peroxide-generating enzyme that uses a D-amino acid as a substrate. We hypothesized that DAO may protect against bacterial infection, because hydrogen peroxide is one of the most important molecules in the antibacterial defense systems in mammals. We show here that DAO suppressed the growth of Staphylococcus aureus in a manner that depended on the concentration of DAO and D-amino acid in vitro. Addition of catalase abolished the bacteriostatic activity of DAO. Although DAO plus D-Ala showed less bactericidal activity, addition of myeloperoxidase (MPO) greatly enhanced the bactericidal activity of DAO. Furthermore, DAO was able to utilize bacterial lysate, which contains D-Ala derived from peptidoglycan; this could produce hydrogen peroxide with, in the presence of myeloperoxidase, formation of hypochlorous acid. This concerted reaction of DAO and MPO led to the bactericidal action. In vivo experiments showed that DAO(-/-) (mutant) mice were more susceptible to S. aureus infection than were DAO(+/+) (wild-type) mice. These results suggest that DAO, together with myeloperoxidase, may play an important role in antibacterial systems in mammals.
Figures



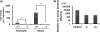
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

