Successful drug development despite adverse preclinical findings part 1: processes to address issues and most important findings
- PMID: 22272031
- PMCID: PMC3234634
- DOI: 10.1293/tox.23.189
Successful drug development despite adverse preclinical findings part 1: processes to address issues and most important findings
Abstract
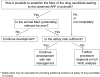
Unexpected adverse preclinical findings (APFs) are not infrequently encountered during drug development. Such APFs can be functional disturbances such as QT prolongation, morphological toxicity or carcinogenicity. The latter is of particular concern in conjunction with equivocal genotoxicity results. The toxicologic pathologist plays an important role in recognizing these effects, in helping to characterize them, to evaluate their risk for man, and in proposing measures to mitigate the risk particularly in early clinical trials. A careful scientific evaluation is crucial while termination of the development of a potentially useful drug must be avoided. This first part of the review discusses processes to address unexpected APFs and provides an overview over typical APFs in particular classes of drugs. If the mode of action (MoA) by which a drug candidate produces an APF is known, this supports evaluation of its relevance for humans. Tailor-made mechanistic studies, when needed, must be planned carefully to test one or several hypotheses regarding the potential MoA and to provide further data for risk evaluation. Safety considerations are based on exposure at no-observed-adverse-effect levels (NOAEL) of the most sensitive and relevant animal species and guide dose escalation in clinical trials. The availability of early markers of toxicity for monitoring of humans adds further safety to clinical studies. Risk evaluation is concluded by a weight of evidence analysis (WoE) with an array of parameters including drug use, medical need and alternatives on the market. In the second part of this review relevant examples of APFs will be discussed in more detail.
Keywords: adverse preclinical finding; hazard identification and characterization; mode of action; risk evaluation and management; safety ratio; weight of evidence.
Figures


Similar articles
-
Successful drug development despite adverse preclinical findings part 2: examples.J Toxicol Pathol. 2010 Dec;23(4):213-34. doi: 10.1293/tox.23.213. Epub 2010 Dec 16. J Toxicol Pathol. 2010. PMID: 22272032 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
In vitro approaches to develop weight of evidence (WoE) and mode of action (MoA) discussions with positive in vitro genotoxicity results.Mutagenesis. 2007 May;22(3):161-75. doi: 10.1093/mutage/gem006. Epub 2007 Mar 16. Mutagenesis. 2007. PMID: 17369606 Review.
-
Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence.J Appl Toxicol. 2014 Jun;34(6):595-606. doi: 10.1002/jat.2984. Epub 2014 Feb 10. J Appl Toxicol. 2014. PMID: 24777878 Free PMC article. Review.
References
-
- International Conference on Harmonization (ICH). ICH harmonized tripartite guideline. Safety pharmacology studies for human pharmaceuticals. S7A. 2000.
-
- Kinter LB, Valentin JP. Safety pharmacology and risk assessment. Fundam Clin Pharmacol. 2002;16:175–182. - PubMed
-
- International Conference on Harmonization (ICH). ICH harmonized tripartite guideline. Nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. S7B. 2005. - PubMed
-
- De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–597. - PubMed
LinkOut - more resources
Full Text Sources
