Prediction of lysine ubiquitylation with ensemble classifier and feature selection
- PMID: 22272076
- PMCID: PMC3257073
- DOI: 10.3390/ijms12128347
Prediction of lysine ubiquitylation with ensemble classifier and feature selection
Abstract
Ubiquitylation is an important process of post-translational modification. Correct identification of protein lysine ubiquitylation sites is of fundamental importance to understand the molecular mechanism of lysine ubiquitylation in biological systems. This paper develops a novel computational method to effectively identify the lysine ubiquitylation sites based on the ensemble approach. In the proposed method, 468 ubiquitylation sites from 323 proteins retrieved from the Swiss-Prot database were encoded into feature vectors by using four kinds of protein sequences information. An effective feature selection method was then applied to extract informative feature subsets. After different feature subsets were obtained by setting different starting points in the search procedure, they were used to train multiple random forests classifiers and then aggregated into a consensus classifier by majority voting. Evaluated by jackknife tests and independent tests respectively, the accuracy of the proposed predictor reached 76.82% for the training dataset and 79.16% for the test dataset, indicating that this predictor is a useful tool to predict lysine ubiquitylation sites. Furthermore, site-specific feature analysis was performed and it was shown that ubiquitylation is intimately correlated with the features of its surrounding sites in addition to features derived from the lysine site itself. The feature selection method is available upon request.
Keywords: ensemble classifier; lysine ubiquitylation sites; support vector machine; ubiquitylation.
Figures


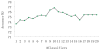
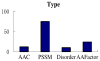
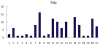
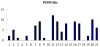
References
-
- Pickart C.M. Ubiquitin enters the new millennium. Mol. Cell. 2001;8:499–504. - PubMed
-
- Aguilar R.C., Wendland B. Ubiquitin: Not just for proteasomes anymore. Curr. Opin. Cell Biol. 2003;15:184–190. - PubMed
-
- Saghatelian A., Cravatt B.F. Assignment of protein function in the postgenomic era. Nat. Chem. Biol. 2005;1:130–142. - PubMed
-
- Herrmann J., Lerman L.O., Lerman A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ. Res. 2007;100:1276–1291. - PubMed
-
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquiti-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

