Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine
- PMID: 22272189
- PMCID: PMC3260300
- DOI: 10.1371/journal.pmed.1001157
Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine
Abstract
Background: The RTS,S malaria vaccine may soon be licensed. Models of impact of such vaccines have mainly considered deployment via the World Health Organization's Expanded Programme on Immunization (EPI) in areas of stable endemic transmission of Plasmodium falciparum, and have been calibrated for such settings. Their applicability to low transmission settings is unclear. Evaluations of the efficiency of different deployment strategies in diverse settings should consider uncertainties in model structure.
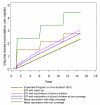
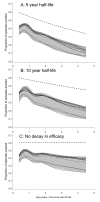
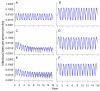
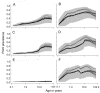
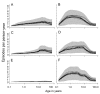
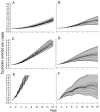
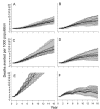
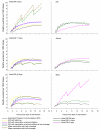
Methods and findings: An ensemble of 14 individual-based stochastic simulation models of P. falciparum dynamics, with differing assumptions about immune decay, transmission heterogeneity, and treatment access, was constructed. After fitting to an extensive library of field data, each model was used to predict the likely health benefits of RTS,S deployment, via EPI (with or without catch-up vaccinations), supplementary vaccination of school-age children, or mass vaccination every 5 y. Settings with seasonally varying transmission, with overall pre-intervention entomological inoculation rates (EIRs) of two, 11, and 20 infectious bites per person per annum, were considered. Predicted benefits of EPI vaccination programs over the simulated 14-y time horizon were dependent on duration of protection. Nevertheless, EPI strategies (with an initial catch-up phase) averted the most deaths per dose at the higher EIRs, although model uncertainty increased with EIR. At two infectious bites per person per annum, mass vaccination strategies substantially reduced transmission, leading to much greater health effects per dose, even at modest coverage.
Conclusions: In higher transmission settings, EPI strategies will be most efficient, but vaccination additional to the EPI in targeted low transmission settings, even at modest coverage, might be more efficient than national-level vaccination of infants. The feasibility and economics of mass vaccination, and the circumstances under which vaccination will avert epidemics, remain unclear. The approach of using an ensemble of models provides more secure conclusions than a single-model approach, and suggests greater confidence in predictions of health effects for lower transmission settings than for higher ones.
Conflict of interest statement
Thomas A. Smith is on the Editorial Board of
Figures

References
-
- London School of Hygiene and Tropical Medicine Public Health Forum. Malaria: waiting for the vaccine. London: Wiley; 1992.
-
- Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–2544. - PubMed
-
- Ballou WR. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 2009;31:492–500. - PubMed
-
- Smith T, Killeen GF, Maire N, Ross A, Molineaux L, et al. Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: overview. Am J Trop Med Hyg. 2006;75:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

