Comparison of Two Ultrasmall Superparamagnetic Iron Oxides on Cytotoxicity and MR Imaging of Tumors
- PMID: 22272221
- PMCID: PMC3263518
- DOI: 10.7150/thno.3462
Comparison of Two Ultrasmall Superparamagnetic Iron Oxides on Cytotoxicity and MR Imaging of Tumors
Abstract
Purpose: This study was performed to compare the cytotoxicity and magnetic resonance (MR) contrast in diverse cultured cells and xenograft tumors models of two ultra-small superparamagnetic iron oxides (USPIOs), thermally cross-linked superparamagnetic iron oxide nanoparticles (TCL-SPION) and monocrystalline iron oxide nanoparticles (MION-47).
Materials and methods: Transmission electron microscopy (TEM) images and R(2) relaxivity values of the TCL-SPION and MION-47 were obtained and the cell viability and cell growth velocity of treated and untreated human fibroblasts and human umbilical vein endothelial cells (HUVEC) were evaluated. The effect of TCL-SPION and MION-47 on the secretion of interlukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), the production of nitric oxides and the mitochondrial membrane potentials in murine macrophage cells (RAW264.7) was compared. Human hepatocellular carcinoma cells (HepG2, 5x10(5)) were subcutaneously injected into nude mice (BALB/c) and in vivo MR imaging of tumors before and after injection with TCL-SPION or MION-47 (12.5 mg Fe/kg) was performed on a 1.5 Tesla MRI scanner.
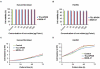
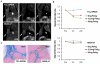
Results: On TEM images, the average core diameter of TCL-SPION was 9 nm whereas that of MION-47 was 5 nm. TCL- SPION (345.0 ± 6.2 mM(-1)sec(-1)) had higher relaxivity (R(2)) than MION-47 (130.7 ± 1.1 mM(-1)sec(-1)). Significant changes in cell viability and growth were not found in human fibroblasts and HUVEC exposed to TCL-SPION and MION-47. However, IL-6 and TNF-α secretions increased dose-dependently and significantly in the macrophages treated with MION-47 or TCL-SPION. TCL-SPION had a lower stimulatory effect on IL-6 secretions than did MION-47 (P <0.05) and nitric oxides were produced in the macrophages by MION-47 but not TCL-SPION. A change in the mitochondrial membrane potential of the macrophages was observed 24 hours after the exposure, and MION-47 induced more collapses of the mitochondrial membrane potential than did TCL-SPION. In the in vivo MR imaging, 33.0 ± 1.3% and 7.5 ± 0.4% signal intensity decrease on T(2)*-weighted images was observed in the tumors injected with TCL-SPION and MION-47, respectively.
Conclusion: Due to the modified surface properties and larger core size of its iron oxide nanoparticles, TCL-SPION achieves lower cytotoxicity and better tumor MR contrast than MION-47. Our study suggests that TCL-SPION may be used as a new platform for tumor imaging and therapy monitoring.
Keywords: MION-47; Magnetic resonance imaging; TCL-SPION; Tumor targeting.; Ultra-small superparamagnetic iron oxides.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures

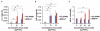

Similar articles
-
MRI of breast tumor initiating cells using the extra domain-B of fibronectin targeting nanoparticles.Theranostics. 2014 Jun 10;4(8):845-57. doi: 10.7150/thno.8343. eCollection 2014. Theranostics. 2014. PMID: 24955145 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate.2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641831 Free Books & Documents. Review.
-
Thermally cross-linked superparamagnetic iron oxide nanoparticle-A10 RNA aptamer-doxorubicin conjugate.2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641568 Free Books & Documents. Review.
-
Thermally cross-linked superparamagnetic iron oxide nanoparticles: synthesis and application as a dual imaging probe for cancer in vivo.J Am Chem Soc. 2007 Oct 24;129(42):12739-45. doi: 10.1021/ja072210i. Epub 2007 Sep 25. J Am Chem Soc. 2007. PMID: 17892287
-
Integrin-targeting thermally cross-linked superparamagnetic iron oxide nanoparticles for combined cancer imaging and drug delivery.Nanotechnology. 2010 Oct 15;21(41):415102. doi: 10.1088/0957-4484/21/41/415102. Epub 2010 Sep 17. Nanotechnology. 2010. PMID: 20852354
Cited by
-
Theranostics for Breast Cancer Stem Cells.Adv Exp Med Biol. 2021;1187:267-281. doi: 10.1007/978-981-32-9620-6_13. Adv Exp Med Biol. 2021. PMID: 33983583
-
Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy.Pharmaceutics. 2021 Jul 28;13(8):1167. doi: 10.3390/pharmaceutics13081167. Pharmaceutics. 2021. PMID: 34452128 Free PMC article. Review.
-
Surface impact on nanoparticle-based magnetic resonance imaging contrast agents.Theranostics. 2018 Apr 3;8(9):2521-2548. doi: 10.7150/thno.23789. eCollection 2018. Theranostics. 2018. PMID: 29721097 Free PMC article. Review.
-
Nanoparticle Effects on Stress Response Pathways and Nanoparticle-Protein Interactions.Int J Mol Sci. 2022 Jul 19;23(14):7962. doi: 10.3390/ijms23147962. Int J Mol Sci. 2022. PMID: 35887304 Free PMC article. Review.
-
MRI of breast tumor initiating cells using the extra domain-B of fibronectin targeting nanoparticles.Theranostics. 2014 Jun 10;4(8):845-57. doi: 10.7150/thno.8343. eCollection 2014. Theranostics. 2014. PMID: 24955145 Free PMC article.
References
-
- Benderbous S, Corot C, Jacobs P, Bonnemain B. Superparamagnetic agents: physicochemical characteristics and preclinical imaging evaluation. Acad Radiol. 1996;3(Suppl 2):S292–4. - PubMed
-
- Huang P, Li Z, Lin J, Yang D, Gao G, Xu C, Bao L, Zhang CL, Wang K, Song H, Hu HY, Cui DX. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447–58. - PubMed
-
- Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9(Suppl 2):S304–6. - PubMed
-
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–30. - PubMed
-
- Leite FP TD, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

