Multi-parametric analysis and modeling of relationships between mitochondrial morphology and apoptosis
- PMID: 22272225
- PMCID: PMC3260148
- DOI: 10.1371/journal.pone.0028694
Multi-parametric analysis and modeling of relationships between mitochondrial morphology and apoptosis
Abstract
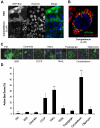
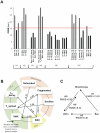
Mitochondria exist as a network of interconnected organelles undergoing constant fission and fusion. Current approaches to study mitochondrial morphology are limited by low data sampling coupled with manual identification and classification of complex morphological phenotypes. Here we propose an integrated mechanistic and data-driven modeling approach to analyze heterogeneous, quantified datasets and infer relations between mitochondrial morphology and apoptotic events. We initially performed high-content, multi-parametric measurements of mitochondrial morphological, apoptotic, and energetic states by high-resolution imaging of human breast carcinoma MCF-7 cells. Subsequently, decision tree-based analysis was used to automatically classify networked, fragmented, and swollen mitochondrial subpopulations, at the single-cell level and within cell populations. Our results revealed subtle but significant differences in morphology class distributions in response to various apoptotic stimuli. Furthermore, key mitochondrial functional parameters including mitochondrial membrane potential and Bax activation, were measured under matched conditions. Data-driven fuzzy logic modeling was used to explore the non-linear relationships between mitochondrial morphology and apoptotic signaling, combining morphological and functional data as a single model. Modeling results are in accordance with previous studies, where Bax regulates mitochondrial fragmentation, and mitochondrial morphology influences mitochondrial membrane potential. In summary, we established and validated a platform for mitochondrial morphological and functional analysis that can be readily extended with additional datasets. We further discuss the benefits of a flexible systematic approach for elucidating specific and general relationships between mitochondrial morphology and apoptosis.
Conflict of interest statement
Figures




Similar articles
-
Bax/Bak-dependent, Drp1-independent Targeting of X-linked Inhibitor of Apoptosis Protein (XIAP) into Inner Mitochondrial Compartments Counteracts Smac/DIABLO-dependent Effector Caspase Activation.J Biol Chem. 2015 Sep 4;290(36):22005-18. doi: 10.1074/jbc.M115.643064. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134559 Free PMC article.
-
Defective mitochondrial fusion, altered respiratory function, and distorted cristae structure in skin fibroblasts with heterozygous OPA1 mutations.Biochim Biophys Acta. 2012 Oct;1822(10):1570-80. doi: 10.1016/j.bbadis.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2012. PMID: 22800932
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
The mitochondrial death pathway: a promising therapeutic target in diseases.J Cell Mol Med. 2009 Jun;13(6):1004-33. doi: 10.1111/j.1582-4934.2009.00697.x. Epub 2009 Feb 9. J Cell Mol Med. 2009. PMID: 19220575 Free PMC article. Review.
-
[Mitochondrial dynamics during apoptosis].Med Sci (Paris). 2010 Oct;26(10):830-5. doi: 10.1051/medsci/20102610830. Med Sci (Paris). 2010. PMID: 20929673 Review. French.
Cited by
-
Quantitative analysis of mitochondrial morphology and membrane potential in living cells using high-content imaging, machine learning, and morphological binning.Biochim Biophys Acta. 2015 Feb;1853(2):348-60. doi: 10.1016/j.bbamcr.2014.11.002. Epub 2014 Nov 13. Biochim Biophys Acta. 2015. PMID: 25447550 Free PMC article.
-
Three-dimensional structure of axonal mitochondria reflects the age of drosophila.Neural Regen Res. 2013 Mar 5;8(7):616-21. doi: 10.3969/j.issn.1673-5374.2013.07.005. Neural Regen Res. 2013. PMID: 25206706 Free PMC article.
-
Machine learning-based classification of mitochondrial morphology in primary neurons and brain.Sci Rep. 2021 Mar 4;11(1):5133. doi: 10.1038/s41598-021-84528-8. Sci Rep. 2021. PMID: 33664336 Free PMC article.
-
In vivo imaging of drug-induced mitochondrial outer membrane permeabilization at single-cell resolution.Cancer Res. 2012 Jun 15;72(12):2949-56. doi: 10.1158/0008-5472.CAN-11-4096. Epub 2012 Apr 13. Cancer Res. 2012. PMID: 22505651 Free PMC article.
-
Deep Analysis of Mitochondria and Cell Health Using Machine Learning.Sci Rep. 2018 Nov 5;8(1):16354. doi: 10.1038/s41598-018-34455-y. Sci Rep. 2018. PMID: 30397207 Free PMC article.
References
-
- Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochimica et biophysica acta. 2006;1757:1297–1300. - PubMed
-
- Henry-Mowatt J, Dive C, Martinou J, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. - PubMed
-
- Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microscopy research and technique. 1994;27:198–219. - PubMed
-
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, et al. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca 2+ Responses. Science. 1998:1–5. - PubMed
-
- Kuznetsov AV, Usson Y, Leverve X, Margreiter R. Subcellular heterogeneity of mitochondrial function and dysfunction: evidence obtained by confocal imaging. Molecular and cellular biochemistry. 2004;256–257:359–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

