Angiopoietin 2 alters pancreatic vascularization in diabetic conditions
- PMID: 22272235
- PMCID: PMC3260141
- DOI: 10.1371/journal.pone.0029438
Angiopoietin 2 alters pancreatic vascularization in diabetic conditions
Abstract
Aims/hypothesis: Islet vascularization, by controlling beta-cell mass expansion in response to increased insulin demand, is implicated in the progression to glucose intolerance and type 2 diabetes. We investigated how hyperglycaemia impairs expansion and differentiation of the growing pancreas. We have grafted xenogenic (avian) embryonic pancreas in severe combined immuno-deficient (SCID) mouse and analyzed endocrine and endothelial development in hyperglycaemic compared to normoglycaemic conditions.
Methods: 14 dpi chicken pancreases were grafted under the kidney capsule of normoglycaemic or hyperglycaemic, streptozotocin-induced, SCID mice and analyzed two weeks later. Vascularization was analyzed both quantitatively and qualitatively using either in situ hybridization with both mouse- and chick-specific RNA probes for VEGFR2 or immunohistochemistry with an antibody to nestin, a marker of endothelial cells that is specific for murine cells. To inhibit angiopoietin 2 (Ang2), SCID mice were treated with 4 mg/kg IP L1-10 twice/week.
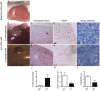
Results: In normoglycaemic condition, chicken-derived endocrine and exocrine cells developed well and intragraft vessels were lined with mouse endothelial cells. When pancreases were grafted in hyperglycaemic mice, growth and differentiation of the graft were altered and we observed endothelial discontinuities, large blood-filled spaces. Vessel density was decreased. These major vascular anomalies were associated with strong over-expression of chick-Ang2. To explore the possibility that Ang2 over-expression could be a key step in vascular disorganization induced by hyperglycaemia, we treated mice with L1-10, an Ang-2 specific inhibitor. Inhibition of Ang2 improved vascularization and beta-cell density.
Conclusions: This work highlighted an important role of Ang2 in pancreatic vascular defects induced by hyperglycaemia.
Conflict of interest statement
Figures





References
-
- Eberhard D, Kragl M, Lammert E. ‘Giving and taking’: endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab. 2010;21:457–463. - PubMed
-
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. - PubMed
-
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. - PubMed
-
- Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50:380–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

