HspB2/myotonic dystrophy protein kinase binding protein (MKBP) as a novel molecular chaperone: structural and functional aspects
- PMID: 22272249
- PMCID: PMC3260166
- DOI: 10.1371/journal.pone.0029810
HspB2/myotonic dystrophy protein kinase binding protein (MKBP) as a novel molecular chaperone: structural and functional aspects
Abstract
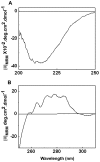
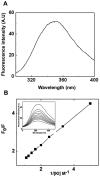
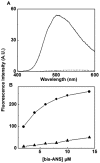
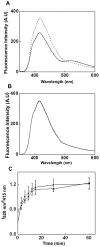
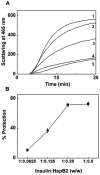
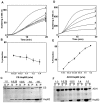
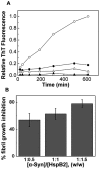
The small heat shock protein, human HspB2, also known as Myotonic Dystrophy Kinase Binding Protein (MKBP), specifically associates with and activates Myotonic Dystrophy Protein Kinase (DMPK), a serine/threonine protein kinase that plays an important role in maintaining muscle structure and function. The structure and function of HspB2 are not well understood. We have cloned and expressed the protein in E.coli and purified it to homogeneity. Far-UV circular dichroic spectrum of the recombinant HspB2 shows a β-sheet structure. Fluorescence spectroscopic studies show that the sole tryptophan residue at the 130(th) position is almost completely solvent-exposed. Bis-ANS binding shows that though HspB2 exhibits accessible hydrophobic surfaces, it is significantly less than that exhibited by another well characterized small HSP, αB-crystallin. Sedimentation velocity measurements show that the protein exhibits concentration-dependent oligomerization. Fluorescence resonance energy transfer study shows that HspB2 oligomers exchange subunits. Interestingly, HspB2 exhibits target protein-dependent chaperone-like activity: it exhibits significant chaperone-like activity towards dithiothreitol (DTT)-induced aggregation of insulin and heat-induced aggregation of alcohol dehydrogenase, but only partially prevents the heat-induced aggregation of citrate synthase, co-precipitating with the target protein. It also significantly prevents the ordered amyloid fibril formation of α-synuclein. Thus, our study, for the first time, provides biophysical characterization on the structural aspects of HspB2, and shows that it exhibits target protein-dependent chaperone-like activity.
Conflict of interest statement
Figures



Similar articles
-
The small heat-shock proteins HSPB2 and HSPB3 form well-defined heterooligomers in a unique 3 to 1 subunit ratio.J Mol Biol. 2009 Nov 13;393(5):1022-32. doi: 10.1016/j.jmb.2009.08.052. Epub 2009 Aug 26. J Mol Biol. 2009. PMID: 19715703
-
Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant.J Mol Biol. 2008 Jan 25;375(4):1040-51. doi: 10.1016/j.jmb.2007.11.019. Epub 2007 Nov 13. J Mol Biol. 2008. PMID: 18061612
-
Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation.J Biol Chem. 2000 Jan 14;275(2):1095-104. doi: 10.1074/jbc.275.2.1095. J Biol Chem. 2000. PMID: 10625651
-
HSPB2/MKBP, a novel and unique member of the small heat-shock protein family.J Neurosci Res. 2008 Aug 1;86(10):2125-33. doi: 10.1002/jnr.21682. J Neurosci Res. 2008. PMID: 18615620 Review.
-
Structural perturbation of alpha-crystallin and its chaperone-like activity.Int J Biol Macromol. 1998 May-Jun;22(3-4):271-81. doi: 10.1016/s0141-8130(98)00025-7. Int J Biol Macromol. 1998. PMID: 9650082 Review.
Cited by
-
HSPA1A-independent suppression of PARK2 C289G protein aggregation by human small heat shock proteins.Mol Cell Biol. 2014 Oct 1;34(19):3570-8. doi: 10.1128/MCB.00698-14. Epub 2014 Jul 14. Mol Cell Biol. 2014. PMID: 25022755 Free PMC article.
-
αB-crystallin and HspB2 deficiency is protective from diet-induced glucose intolerance.Genom Data. 2016 May 13;9:10-7. doi: 10.1016/j.gdata.2016.03.010. eCollection 2016 Sep. Genom Data. 2016. PMID: 27330996 Free PMC article.
-
Characterization of the Cardiac Overexpression of HSPB2 Reveals Mitochondrial and Myogenic Roles Supported by a Cardiac HspB2 Interactome.PLoS One. 2015 Oct 14;10(10):e0133994. doi: 10.1371/journal.pone.0133994. eCollection 2015. PLoS One. 2015. PMID: 26465331 Free PMC article.
-
Structural aspects of the human small heat shock proteins related to their functional activities.Cell Stress Chaperones. 2020 Jul;25(4):581-591. doi: 10.1007/s12192-020-01093-1. Epub 2020 Apr 6. Cell Stress Chaperones. 2020. PMID: 32253739 Free PMC article. Review.
-
miR-17-5p promotes the invasion and migration of colorectal cancer by regulating HSPB2.J Cancer. 2022 Jan 1;13(3):918-931. doi: 10.7150/jca.65614. eCollection 2022. J Cancer. 2022. PMID: 35154459 Free PMC article.
References
-
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG, King J, Rajnarayan S, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. - PubMed
-
- Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. - PubMed
-
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the alpha-crystallin/small hsp family, closely linked to the alphaB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

