Site-directed mutations and the polymorphic variant Ala160Thr in the human thromboxane receptor uncover a structural role for transmembrane helix 4
- PMID: 22272267
- PMCID: PMC3260207
- DOI: 10.1371/journal.pone.0029996
Site-directed mutations and the polymorphic variant Ala160Thr in the human thromboxane receptor uncover a structural role for transmembrane helix 4
Abstract
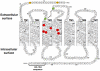
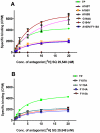
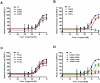
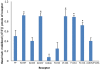
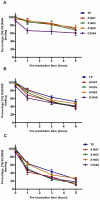
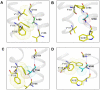
The human thromboxane A2 receptor (TP), belongs to the prostanoid subfamily of Class A GPCRs and mediates vasoconstriction and promotes thrombosis on binding to thromboxane (TXA2). In Class A GPCRs, transmembrane (TM) helix 4 appears to be a hot spot for non-synonymous single nucleotide polymorphic (nsSNP) variants. Interestingly, A160T is a novel nsSNP variant with unknown structure and function. Additionally, within this helix in TP, Ala160(4.53) is highly conserved as is Gly164(4.57). Here we target Ala160(4.53) and Gly164(4.57) in the TP for detailed structure-function analysis. Amino acid replacements with smaller residues, A160S and G164A mutants, were tolerated, while bulkier beta-branched replacements, A160T and A160V showed a significant decrease in receptor expression (Bmax). The nsSNP variant A160T displayed significant agonist-independent activity (constitutive activity). Guided by molecular modeling, a series of compensatory mutations were made on TM3, in order to accommodate the bulkier replacements on TM4. The A160V/F115A double mutant showed a moderate increase in expression level compared to either A160V or F115A single mutants. Thermal activity assays showed decrease in receptor stability in the order, wild type>A160S>A160V>A160T>G164A, with G164A being the least stable. Our study reveals that Ala160(4.53) and Gly164(4.57) in the TP play critical structural roles in packing of TM3 and TM4 helices. Naturally occurring mutations in conjunction with site-directed replacements can serve as powerful tools in assessing the importance of regional helix-helix interactions.
Conflict of interest statement
Figures


Similar articles
-
High-level expression, purification and characterization of a constitutively active thromboxane A2 receptor polymorphic variant.PLoS One. 2013 Sep 23;8(9):e76481. doi: 10.1371/journal.pone.0076481. eCollection 2013. PLoS One. 2013. PMID: 24086743 Free PMC article.
-
Inverse agonism of SQ 29,548 and Ramatroban on Thromboxane A2 receptor.PLoS One. 2014 Jan 23;9(1):e85937. doi: 10.1371/journal.pone.0085937. eCollection 2014. PLoS One. 2014. PMID: 24465800 Free PMC article.
-
Platelet dysfunction associated with the novel Trp29Cys thromboxane A₂ receptor variant.J Thromb Haemost. 2013 Mar;11(3):547-54. doi: 10.1111/jth.12117. J Thromb Haemost. 2013. PMID: 23279270
-
A novel thromboxane A2 receptor D304N variant that abrogates ligand binding in a patient with a bleeding diathesis.Blood. 2010 Jan 14;115(2):363-9. doi: 10.1182/blood-2009-08-236976. Epub 2009 Oct 14. Blood. 2010. PMID: 19828703 Free PMC article.
-
Thromboxane A2 receptor and MaxiK-channel intimate interaction supports channel trans-inhibition independent of G-protein activation.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):19096-101. doi: 10.1073/pnas.1002685107. Epub 2010 Oct 19. Proc Natl Acad Sci U S A. 2010. PMID: 20959415 Free PMC article.
Cited by
-
New insights into structural determinants for prostanoid thromboxane A2 receptor- and prostacyclin receptor-G protein coupling.Mol Cell Biol. 2013 Jan;33(2):184-93. doi: 10.1128/MCB.00725-12. Epub 2012 Oct 29. Mol Cell Biol. 2013. PMID: 23109431 Free PMC article.
-
Thromboxane receptor hyper-responsiveness in hypoxic pulmonary hypertension requires serine 324.Br J Pharmacol. 2014 Feb;171(3):676-87. doi: 10.1111/bph.12487. Br J Pharmacol. 2014. PMID: 24490858 Free PMC article.
-
Characterization of Adenylyl Cyclase Isoform 6 Residues Interacting with Forskolin.Biology (Basel). 2023 Apr 10;12(4):572. doi: 10.3390/biology12040572. Biology (Basel). 2023. PMID: 37106773 Free PMC article.
-
High-level expression, purification and characterization of a constitutively active thromboxane A2 receptor polymorphic variant.PLoS One. 2013 Sep 23;8(9):e76481. doi: 10.1371/journal.pone.0076481. eCollection 2013. PLoS One. 2013. PMID: 24086743 Free PMC article.
-
Evolution of the genetic code by incorporation of amino acids that improved or changed protein function.J Mol Evol. 2013 Oct;77(4):134-58. doi: 10.1007/s00239-013-9567-y. Epub 2013 Jun 7. J Mol Evol. 2013. PMID: 23743924
References
-
- Kinsella BT. Thromboxane A2 signalling in humans: a ‘Tail’ of two receptors. Biochem Soc Trans. 2001;29:641–654. - PubMed
-
- Kim SH, Choi JH, Park HS, Holloway JW, Lee SK, et al. Association of thromboxane A2 receptor gene polymorphism with the phenotype of acetyl salicylic acid-intolerant asthma. Clin Exp Allergy. 2005;35:585–590. - PubMed
-
- Hong SJ, Lee SY, Kim HB, Kim JH, Kim BS, et al. IL-5 and thromboxane A2 receptor gene polymorphisms are associated with decreased pulmonary function in Korean children with atopic asthma. J Allergy Clin Immunol. 2005;115:758–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

