Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery
- PMID: 22272269
- PMCID: PMC3260195
- DOI: 10.1371/journal.pone.0030010
Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery
Abstract
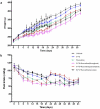
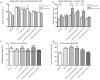
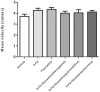
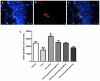
5-Fluorouracil (5-FU) is a cytostatic drug associated with chemotherapy-induced cognitive impairments that many cancer patients experience after treatment. Previous work in rodents has shown that 5-FU reduces hippocampal cell proliferation, a possible mechanism for the observed cognitive impairment, and that both effects can be reversed by co-administration of the antidepressant, fluoxetine. In the present study we investigate the optimum time for administration of fluoxetine to reverse or prevent the cognitive and cellular effects of 5-FU. Male Lister-hooded rats received 5 injections of 5-FU (25 mg/kg, i.p.) over 2 weeks. Some rats were co-administered with fluoxetine (10 mg/kg/day, in drinking water) for 3 weeks before and during (preventative) or after (recovery) 5-FU treatment or both time periods (throughout). Spatial memory was tested using the novel location recognition (NLR) test and proliferation and survival of hippocampal cells was quantified using immunohistochemistry. 5-FU-treated rats showed cognitive impairment in the NLR task and a reduction in cell proliferation and survival in the subgranular zone of the dentate gyrus, compared to saline treated controls. These impairments were still seen for rats administered fluoxetine after 5-FU treatment, but were not present when fluoxetine was administered both before and during 5-FU treatment. The results demonstrate that fluoxetine is able to prevent but not reverse the cognitive and cellular effects of 5-FU. This provides information on the mechanism by which fluoxetine acts to protect against 5-FU and indicates when it would be beneficial to administer the antidepressant to cancer patients.
Conflict of interest statement
Figures


Similar articles
-
Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy.PLoS One. 2017 Jul 10;12(7):e0180650. doi: 10.1371/journal.pone.0180650. eCollection 2017. PLoS One. 2017. PMID: 28700628 Free PMC article.
-
Melatonin attenuates 5-fluorouracil-induced spatial memory and hippocampal neurogenesis impairment in adult rats.Life Sci. 2020 May 1;248:117468. doi: 10.1016/j.lfs.2020.117468. Epub 2020 Feb 25. Life Sci. 2020. PMID: 32105705
-
Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy.Psychopharmacology (Berl). 2011 May;215(1):105-15. doi: 10.1007/s00213-010-2122-2. Epub 2010 Dec 22. Psychopharmacology (Berl). 2011. PMID: 21181126 Free PMC article.
-
Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil.Behav Brain Res. 2010 Mar 17;208(1):112-7. doi: 10.1016/j.bbr.2009.11.017. Epub 2009 Nov 13. Behav Brain Res. 2010. PMID: 19914299
-
5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus.Eur J Neurosci. 2008 Jul;28(2):323-30. doi: 10.1111/j.1460-9568.2008.06325.x. Eur J Neurosci. 2008. PMID: 18702703
Cited by
-
In-vitro and in-vivo studies of two-drug cocktail therapy targeting chemobrain via the Nrf2/NF-κB signaling pathway.J Mol Histol. 2024 Aug;55(4):599-625. doi: 10.1007/s10735-024-10217-1. Epub 2024 Jul 23. J Mol Histol. 2024. PMID: 39042217
-
Structural and functional brain alterations associated with cancer-associated cognitive decline in gastric cancer patients: A preliminary longitudinal neuroimaging study.Brain Behav. 2022 Jan;12(1):e2437. doi: 10.1002/brb3.2437. Epub 2021 Nov 25. Brain Behav. 2022. PMID: 34825514 Free PMC article.
-
Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy.PLoS One. 2017 Jul 10;12(7):e0180650. doi: 10.1371/journal.pone.0180650. eCollection 2017. PLoS One. 2017. PMID: 28700628 Free PMC article.
-
Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know?Semin Oncol. 2013 Dec;40(6):709-25. doi: 10.1053/j.seminoncol.2013.09.006. Semin Oncol. 2013. PMID: 24331192 Free PMC article. Review.
-
Cancer related cognitive impairment: a downside of cancer treatment.Front Oncol. 2024 Apr 23;14:1387251. doi: 10.3389/fonc.2024.1387251. eCollection 2024. Front Oncol. 2024. PMID: 38715789 Free PMC article. Review.
References
-
- Taillibert Sa, Voillery Da, Bernard-Marty Cb. Chemobrain: is systemic chemotherapy neurotoxic? Current Opinion in Oncology. 2007;19:623–627. - PubMed
-
- Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(Suppl 3):S84–90. - PubMed
-
- Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, et al. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients–evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12:279–287. - PubMed
-
- Kreukels BP, van Dam FS, Ridderinkhof KR, Boogerd W, Schagen SB. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer. 2008;8:80–87. - PubMed
-
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

