Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds
- PMID: 22272277
- PMCID: PMC3260182
- DOI: 10.1371/journal.pone.0030083
Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds
Abstract
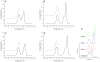
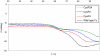
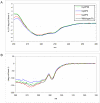
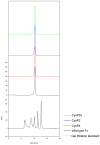
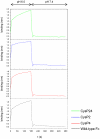
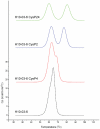
We report the stabilization of the human IgG1 Fc fragment by engineered intradomain disulfide bonds. One of these bonds, which connects the N-terminus of the CH3 domain with the F-strand, led to an increase of the melting temperature of this domain by 10°C as compared to the CH3 domain in the context of the wild-type Fc region. Another engineered disulfide bond, which connects the BC loop of the CH3 domain with the D-strand, resulted in an increase of T(m) of 5°C. Combined in one molecule, both intradomain disulfide bonds led to an increase of the T(m) of about 15°C. All of these mutations had no impact on the thermal stability of the CH2 domain. Importantly, the binding of neonatal Fc receptor was also not influenced by the mutations. Overall, the stabilized CH3 domains described in this report provide an excellent basic scaffold for the engineering of Fc fragments for antigen-binding or other desired additional or improved properties. Additionally, we have introduced the intradomain disulfide bonds into an IgG Fc fragment engineered in C-terminal loops of the CH3 domain for binding to Her2/neu, and observed an increase of the T(m) of the CH3 domain for 7.5°C for CysP4, 15.5°C for CysP2 and 19°C for the CysP2 and CysP4 disulfide bonds combined in one molecule.
Conflict of interest statement
Figures

Similar articles
-
Comprehensive elucidation of the structural and functional roles of engineered disulfide bonds in antibody Fc fragment.J Biol Chem. 2018 Dec 7;293(49):19127-19135. doi: 10.1074/jbc.RA118.005367. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327432 Free PMC article.
-
A C-terminal interdomain disulfide bond significantly stabilizes the Fc fragment of IgG.Arch Biochem Biophys. 2012 Oct 15;526(2):181-7. doi: 10.1016/j.abb.2012.03.024. Epub 2012 Mar 29. Arch Biochem Biophys. 2012. PMID: 22483683
-
Structural differences between glycosylated, disulfide-linked heterodimeric Knob-into-Hole Fc fragment and its homodimeric Knob-Knob and Hole-Hole side products.Protein Eng Des Sel. 2017 Sep 1;30(9):649-656. doi: 10.1093/protein/gzx041. Protein Eng Des Sel. 2017. PMID: 28985438
-
Engineered IgG1-Fc--one fragment to bind them all.Immunol Rev. 2016 Mar;270(1):113-31. doi: 10.1111/imr.12385. Immunol Rev. 2016. PMID: 26864108 Free PMC article. Review.
-
Stellabody: A novel hexamer-promoting mutation for improved IgG potency.Immunol Rev. 2024 Nov;328(1):438-455. doi: 10.1111/imr.13400. Epub 2024 Oct 4. Immunol Rev. 2024. PMID: 39364646 Free PMC article. Review.
Cited by
-
Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen.Vaccine. 2018 Mar 27;36(14):1853-1862. doi: 10.1016/j.vaccine.2018.02.065. Epub 2018 Feb 26. Vaccine. 2018. PMID: 29496347 Free PMC article.
-
Mapping conformational changes on bispecific antigen-binding biotherapeutic by covalent labeling and mass spectrometry.J Pharm Anal. 2024 Aug;14(8):100966. doi: 10.1016/j.jpha.2024.100966. Epub 2024 Mar 16. J Pharm Anal. 2024. PMID: 39263356 Free PMC article.
-
Comprehensive elucidation of the structural and functional roles of engineered disulfide bonds in antibody Fc fragment.J Biol Chem. 2018 Dec 7;293(49):19127-19135. doi: 10.1074/jbc.RA118.005367. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327432 Free PMC article.
-
A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation.Life (Basel). 2021 Oct 29;11(11):1157. doi: 10.3390/life11111157. Life (Basel). 2021. PMID: 34833033 Free PMC article.
-
An antibody with Fab-constant domains exchanged for a pair of CH3 domains.PLoS One. 2018 Apr 9;13(4):e0195442. doi: 10.1371/journal.pone.0195442. eCollection 2018. PLoS One. 2018. PMID: 29630643 Free PMC article.
References
-
- Huber R, Deisenhofer J, Colman PM, Matsushima M, Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–420. - PubMed
-
- Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschlager M, et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel. 2010;23:289–297. - PubMed
-
- Tischenko VM, Abramov VM, Zav'yalov VP. Investigation of the cooperative structure of Fc fragments from myeloma immunoglobulin G. Biochemistry. 1998;37:5576–5581. - PubMed
-
- Ghirlando R, Lund J, Goodall M, Jefferis R. Glycosylation of human IgG-Fc: influences on structure revealed by differential scanning micro-calorimetry. Immunol Lett. 1999;68:47–52. - PubMed
-
- Demarest SJ, Rogers J, Hansen G. Optimization of the antibody C(H)3 domain by residue frequency analysis of IgG sequences. J Mol Biol. 2004;335:41–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

