The scaffolding protein Dlg1 is a negative regulator of cell-free virus infectivity but not of cell-to-cell HIV-1 transmission in T cells
- PMID: 22272285
- PMCID: PMC3260186
- DOI: 10.1371/journal.pone.0030130
The scaffolding protein Dlg1 is a negative regulator of cell-free virus infectivity but not of cell-to-cell HIV-1 transmission in T cells
Abstract
Background: Cell-to-cell virus transmission of Human immunodeficiency virus type-1 (HIV-1) is predominantly mediated by cellular structures such as the virological synapse (VS). The VS formed between an HIV-1-infected T cell and a target T cell shares features with the immunological synapse (IS). We have previously identified the human homologue of the Drosophila Discs Large (Dlg1) protein as a new cellular partner for the HIV-1 Gag protein and a negative regulator of HIV-1 infectivity. Dlg1, a scaffolding protein plays a key role in clustering protein complexes in the plasma membrane at cellular contacts. It is implicated in IS formation and T cell signaling, but its role in HIV-1 cell-to-cell transmission was not studied before.
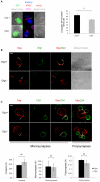
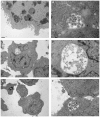
Methodology/principal findings: Kinetics of HIV-1 infection in Dlg1-depleted Jurkat T cells show that Dlg1 modulates the replication of HIV-1. Single-cycle infectivity tests show that this modulation does not take place during early steps of the HIV-1 life cycle. Immunofluorescence studies of Dlg1-depleted Jurkat T cells show that while Dlg1 depletion affects IS formation, it does not affect HIV-1-induced VS formation. Co-culture assays and quantitative cell-to-cell HIV-1 transfer analyses show that Dlg1 depletion does not modify transfer of HIV-1 material from infected to target T cells, or HIV-1 transmission leading to productive infection via cell contact. Dlg1 depletion results in increased virus yield and infectivity of the viral particles produced. Particles with increased infectivity present an increase in their cholesterol content and during the first hours of T cell infection these particles induce higher accumulation of total HIV-1 DNA.
Conclusion: Despite its role in the IS formation, Dlg1 does not affect the VS and cell-to-cell spread of HIV-1, but plays a role in HIV-1 cell-free virus transmission. We propose that the effect of Dlg1 on HIV-1 infectivity is at the stage of virus entry.
Conflict of interest statement
Figures




Similar articles
-
Human Discs Large is a new negative regulator of human immunodeficiency virus-1 infectivity.Mol Biol Cell. 2009 Jan;20(1):498-508. doi: 10.1091/mbc.e08-02-0189. Epub 2008 Oct 22. Mol Biol Cell. 2009. PMID: 18946087 Free PMC article.
-
Knockdown of synapse-associated protein Dlg1 reduces syncytium formation induced by human T-cell leukemia virus type 1.Virus Genes. 2008 Aug;37(1):9-15. doi: 10.1007/s11262-008-0234-0. Epub 2008 May 7. Virus Genes. 2008. PMID: 18461433
-
Nef does not inhibit F-actin remodelling and HIV-1 cell-cell transmission at the T lymphocyte virological synapse.Eur J Cell Biol. 2011 Nov;90(11):913-21. doi: 10.1016/j.ejcb.2010.09.010. Epub 2010 Nov 1. Eur J Cell Biol. 2011. PMID: 21044805
-
Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1.Methods. 2011 Jan;53(1):27-33. doi: 10.1016/j.ymeth.2010.04.002. Epub 2010 Apr 9. Methods. 2011. PMID: 20382227 Review.
-
Masters of manipulation: Viral modulation of the immunological synapse.Cell Microbiol. 2018 Oct;20(10):e12944. doi: 10.1111/cmi.12944. Cell Microbiol. 2018. PMID: 30123959 Free PMC article. Review.
Cited by
-
Single-Virus Tracking: From Imaging Methodologies to Virological Applications.Chem Rev. 2020 Feb 12;120(3):1936-1979. doi: 10.1021/acs.chemrev.9b00692. Epub 2020 Jan 17. Chem Rev. 2020. PMID: 31951121 Free PMC article. Review.
-
Electron microscopy: essentials for viral structure, morphogenesis and rapid diagnosis.Sci China Life Sci. 2013 May;56(5):421-30. doi: 10.1007/s11427-013-4476-2. Epub 2013 May 1. Sci China Life Sci. 2013. PMID: 23633074 Free PMC article. Review.
-
DLG1 promotes the antiviral innate immune response by inhibiting p62-mediated autophagic degradation of IKKε.J Virol. 2023 Dec 21;97(12):e0150123. doi: 10.1128/jvi.01501-23. Epub 2023 Nov 20. J Virol. 2023. PMID: 37982618 Free PMC article.
-
Molecular Profiling of VGluT1 AND VGluT2 Ventral Subiculum to Nucleus Accumbens Shell Projections.Neurochem Res. 2023 Aug;48(8):2490-2501. doi: 10.1007/s11064-023-03921-z. Epub 2023 Apr 5. Neurochem Res. 2023. PMID: 37017888
-
Multiple Inhibitory Factors Act in the Late Phase of HIV-1 Replication: a Systematic Review of the Literature.Microbiol Mol Biol Rev. 2018 Jan 10;82(1):e00051-17. doi: 10.1128/MMBR.00051-17. Print 2018 Mar. Microbiol Mol Biol Rev. 2018. PMID: 29321222 Free PMC article.
References
-
- Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, et al. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem. 2004;279:51305–51314. - PubMed
-
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

