Allosteric antagonism of insect odorant receptor ion channels
- PMID: 22272331
- PMCID: PMC3260273
- DOI: 10.1371/journal.pone.0030304
Allosteric antagonism of insect odorant receptor ion channels
Abstract
Background: At a molecular level, insects utilize members of several highly divergent and unrelated families of cell-surface chemosensory receptors for detection of volatile odorants. Most odors are detected via a family of odorant receptors (ORs), which form heteromeric complexes consisting of a well-conserved OR co-receptor (Orco) ion channel and a non-conserved tuning OR that provides coding specificity to each complex. Orco functions as a non-selective cation channel and is expressed in the majority of olfactory receptor neurons (ORNs). As the destructive behaviors of many insects are principally driven by olfaction, Orco represents a novel target for behavior-based control strategies. While many natural and synthetic odorants have been shown to agonize Orco/Or complexes, only a single direct Orco modulator, VUAA1, has been described. In an effort to identify additional Orco modulators, we have investigated the structure/activity relationships around VUAA1.
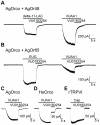
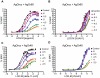
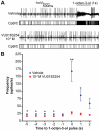
Results: A search of our compound library identified several VUAA1 analogs that were selected for evaluation against HEK cells expressing Orco from the malaria vector Anopheles gambiae (AgOrco). While the majority of compounds displayed no activity, many of these analogs possess no intrinsic efficacy, but instead, act as competitive VUAA1 antagonists. Using calcium mobilization assays, patch clamp electrophysiology, and single sensillum in vivo recording, we demonstrate that one such candidate, VU0183254, is a specific allosteric modulator of OR signaling, capable of broadly inhibiting odor-mediated OR complex activation.
Conclusions: We have described and characterized the first Orco antagonist, that is capable of non-competitively inhibiting odorant-evoked activation of OR complexes, thereby providing additional insight into the structure/function of this unique family of ligand-gated ion channels. While Orco antagonists are likely to have limited utility in insect control programs, they represent important pharmacological tools that will facilitate the investigation of the molecular mechanisms underlying insect olfactory signal transduction.
Conflict of interest statement
Figures


References
-
- Gilliot C. Entomology. 2005. 3rd Edition.
-
- Sanchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity. 2009;103:208–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

