Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: a randomized controlled trial
- PMID: 22272750
- PMCID: PMC3292842
- DOI: 10.1186/1741-7015-10-8
Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: a randomized controlled trial
Abstract
Background: Iron deficiency without anemia is related to adverse symptoms that can be relieved by supplementation. Since a blood donation can induce such an iron deficiency, we investigated the clinical impact of iron treatment after a blood donation.
Methods: One week after donation, we randomly assigned 154 female donors with iron deficiency without anemia, aged below 50 years, to a four-week oral treatment of ferrous sulfate versus a placebo. The main outcome was the change in the level of fatigue before and after the intervention. Aerobic capacity, mood disorder, quality of life, compliance and adverse events were also evaluated. Hemoglobin and ferritin were used as biological markers.
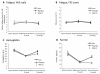
Results: The effect of the treatment from baseline to four weeks of iron treatment was an increase in hemoglobin and ferritin levels to 5.2 g/L (P < 0.01) and 14.8 ng/mL (P < 0.01), respectively. No significant clinical effect was observed for fatigue (-0.15 points, 95% confidence interval -0.9 points to 0.6 points, P = 0.697) or for other outcomes. Compliance and interruption for side effects was similar in both groups. Additionally, blood donation did not induce overt symptoms of fatigue in spite of the significant biological changes it produces.
Conclusions: These data are valuable as they enable us to conclude that donors with iron deficiency without anemia after a blood donation would not clinically benefit from iron supplementation.
Trial registration: ClinicalTrials.gov: NCT00981877.
Figures
References
-
- Beutler E, Larsh SE, Gurney CW. Iron therapy in chronically fatigued, nonanemic women: a double-blind study. Ann Intern Med. 1960;52:378–394. - PubMed
-
- Ballin A, Berar M, Rubinstein U, Kleter Y, Hershkovitz A, Meytes D. Iron state in female adolescents. Am J Dis Child. 1992;146(7):803–805. - PubMed
-
- Patterson AJ, Brown WJ, Roberts DC. Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. Am J Clin Nutr. 2001;20(4):337–342. - PubMed
-
- Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, Bischoff T, de Vevey M, Studer JP, Herzig L, Chapuis C, Tissot J, Pécoud A, Favrat B. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326(7399):1124. doi: 10.1136/bmj.326.7399.1124. - DOI - PMC - PubMed
-
- Brownlie Tt, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75(4):734–742. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical