Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL
- PMID: 22272766
- PMCID: PMC3274638
- DOI: 10.2174/156720212799297137
Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL
Abstract
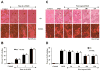
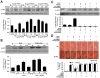
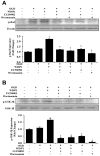
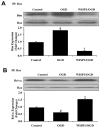
Wnt1 inducible signaling pathway protein 1 (WISP1) is a member of the CCN family of proteins that determine cell growth, cell differentiation, immune system activation, and cell survival in tissues ranging from the cardiovascular-pulmonary system to the reproductive system. Yet, little is known of the role of WISP1 as a neuroprotective entity in the nervous system. Here we demonstrate that WISP1 is present in primary hippocampal neurons during oxidant stress with oxygen-glucose deprivation (OGD). WISP1 expression is significantly enhanced during OGD exposure by the cysteine-rich glycosylated protein Wnt1. Similar to the neuroprotective capabilities known for Wnt1 and its signaling pathways, WISP1 averts neuronal cell injury and apoptotic degeneration during oxidative stress exposure. WISP1 requires activation of phosphoinositide 3-kinase (PI 3-K) and Akt1 pathways to promote neuronal cell survival, since blockade of these pathways abrogates cellular protection. Furthermore, WISP1 through PI 3-K and Akt1 phosphorylates Bad and GSK-3β, minimizes expression of the Bim/Bax complex while increasing the expression of Bclx(L)/Bax complex, and prevents mitochondrial membrane permeability, cytochrome c release, and caspase 3 activation in the presence of oxidant stress. These studies provide novel considerations for the development of WISP1 as an effective and robust therapeutic target not only for neurodegenerative disorders, but also for disease entities throughout the body.
Figures

Similar articles
-
WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death.Cell Signal. 2010 May;22(5):809-20. doi: 10.1016/j.cellsig.2010.01.005. Epub 2010 Jan 13. Cell Signal. 2010. PMID: 20074638 Free PMC article.
-
Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress.Curr Neurovasc Res. 2006 May;3(2):107-17. doi: 10.2174/156720206776875830. Curr Neurovasc Res. 2006. PMID: 16719794 Free PMC article.
-
WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1.Curr Neurovasc Res. 2013 Feb;10(1):54-69. doi: 10.2174/156720213804805945. Curr Neurovasc Res. 2013. PMID: 23151077 Free PMC article.
-
WISP1: Clinical insights for a proliferative and restorative member of the CCN family.Curr Neurovasc Res. 2014;11(4):378-89. doi: 10.2174/1567202611666140912115107. Curr Neurovasc Res. 2014. PMID: 25219658 Free PMC article. Review.
-
Targeting disease through novel pathways of apoptosis and autophagy.Expert Opin Ther Targets. 2012 Dec;16(12):1203-14. doi: 10.1517/14728222.2012.719499. Epub 2012 Aug 27. Expert Opin Ther Targets. 2012. PMID: 22924465 Free PMC article. Review.
Cited by
-
Fufang Duzheng tablet attenuates adjuvant rheumatoid arthritis by inhibiting arthritis inflammation and gut microbiota disturbance in rats.Heliyon. 2024 Jun 7;10(12):e32705. doi: 10.1016/j.heliyon.2024.e32705. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39183834 Free PMC article.
-
Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease.Neural Regen Res. 2015 Apr;10(4):518-28. doi: 10.4103/1673-5374.155427. Neural Regen Res. 2015. PMID: 26170801 Free PMC article. Review.
-
The matricellular protein Drosophila Cellular Communication Network Factor is required for synaptic transmission and female fertility.Genetics. 2023 Mar 2;223(3):iyac190. doi: 10.1093/genetics/iyac190. Genetics. 2023. PMID: 36602539 Free PMC article.
-
WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD.Int J Mol Sci. 2024 Sep 18;25(18):10049. doi: 10.3390/ijms251810049. Int J Mol Sci. 2024. PMID: 39337534 Free PMC article. Review.
-
Neuroserpin Attenuates H2O2-Induced Oxidative Stress in Hippocampal Neurons via AKT and BCL-2 Signaling Pathways.J Mol Neurosci. 2017 Jan;61(1):123-131. doi: 10.1007/s12031-016-0807-7. Epub 2016 Aug 11. J Mol Neurosci. 2017. PMID: 27510267
References
-
- Berschneider B, Konigshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2010;43(3):306–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
