Gender effect on neurodegeneration and myelin markers in an animal model for multiple sclerosis
- PMID: 22272832
- PMCID: PMC3282645
- DOI: 10.1186/1471-2202-13-12
Gender effect on neurodegeneration and myelin markers in an animal model for multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) varies considerably in its incidence and progression in females and males. In spite of clinical evidence, relatively few studies have explored molecular mechanisms possibly involved in gender-related differences. The present study describes possible cellular- and molecular-involved markers which are differentially regulated in male and female rats and result in gender-dependent EAE evolution and progression. Attention was focused on markers of myelination (MBP and PDGFαR) and neuronal distress and/or damage (GABA synthesis enzymes, GAD65 and GAD67, NGF, BDNF and related receptors), in two CNS areas, i.e. spinal cord and cerebellum, which are respectively severely and mildly affected by inflammation and demyelination. Tissues were sampled during acute, relapse/remission and chronic phases and results were analysed by two-way ANOVA.
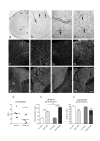
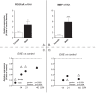
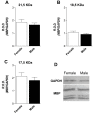
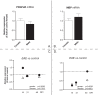
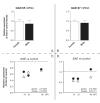
Results: 1. A strong gender-dependent difference in myelin (MBP) and myelin precursor (PDGFαR) marker mRNA expression levels is observed in control animals in the spinal cord, but not in the cerebellum. This is the only gender-dependent difference in the expression level of the indicated markers in healthy animals; 2. both PDGFαR and MBP mRNAs in the spinal cord and MBP in the cerebellum are down-regulated during EAE in gender-dependent manner; 3. in the cerebellum, the expression profile of neuron-associated markers (GAD65, GAD67) is characterized by a substantial down-regulation during the inflammatory phase of the disease, which does not differ between male and female rats (two-way ANOVA); 4. there is an up-regulation of NGF, trkA and p75 mRNA expression in the early phases of the disease (14 and 21 days post-immunization), which is not different between male and female.
Conclusions: It is reported herein that the regulation of markers involved in demyelination and neuroprotection processes occurring during EAE, a well-established MS animal model, is gender- and time-dependent. These findings might contribute to gender- and phase disease-based therapy strategies.
Figures
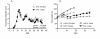
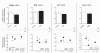
Similar articles
-
Correlation of nitric oxide levels in the cerebellum and spinal cord of experimental autoimmune encephalomyelitis rats with clinical symptoms.Acta Neurobiol Exp (Wars). 2012;72(1):33-9. doi: 10.55782/ane-2012-1878. Acta Neurobiol Exp (Wars). 2012. PMID: 22508082
-
Disruptions to the cerebellar GABAergic system in juvenile guinea pigs following preterm birth.Int J Dev Neurosci. 2018 Apr;65:1-10. doi: 10.1016/j.ijdevneu.2017.10.002. Epub 2017 Oct 9. Int J Dev Neurosci. 2018. PMID: 29024720
-
Cytokine and chemokine alterations in tissue, CSF, and plasma in early presymptomatic phase of experimental allergic encephalomyelitis (EAE), in a rat model of multiple sclerosis.J Neuroinflammation. 2016 Nov 15;13(1):291. doi: 10.1186/s12974-016-0757-6. J Neuroinflammation. 2016. PMID: 27846891 Free PMC article.
-
Oxidative damage and chemokine production dominate days before immune cell infiltration and EAE disease debut.J Neuroinflammation. 2016 Sep 15;13(1):246. doi: 10.1186/s12974-016-0707-3. J Neuroinflammation. 2016. PMID: 27630002 Free PMC article.
-
Expression of glutamate transporters GLT-1 and GLAST in different regions of rat brain during the course of experimental autoimmune encephalomyelitis.Neuroscience. 2008 Jul 31;155(1):45-52. doi: 10.1016/j.neuroscience.2008.05.025. Epub 2008 May 24. Neuroscience. 2008. PMID: 18572325
Cited by
-
Sex, myelin, and clinical characteristics of Parkinson's disease.Front Neurosci. 2023 Sep 13;17:1235524. doi: 10.3389/fnins.2023.1235524. eCollection 2023. Front Neurosci. 2023. PMID: 37781247 Free PMC article.
-
Multikinase Abl/DDR/Src Inhibition Produces Optimal Effects for Tyrosine Kinase Inhibition in Neurodegeneration.Drugs R D. 2019 Jun;19(2):149-166. doi: 10.1007/s40268-019-0266-z. Drugs R D. 2019. PMID: 30919310 Free PMC article.
-
Differential local tissue permissiveness influences the final fate of GPR17-expressing oligodendrocyte precursors in two distinct models of demyelination.Glia. 2018 May;66(5):1118-1130. doi: 10.1002/glia.23305. Epub 2018 Feb 9. Glia. 2018. PMID: 29424466 Free PMC article.
-
Oxidative Stress and Lymphocyte Alterations in Chronic Relapsing Experimental Allergic Encephalomyelitis in the Rat Hippocampus and Protective Effects of an Ethanolamine Phosphate Salt.Mol Neurobiol. 2020 Feb;57(2):860-878. doi: 10.1007/s12035-019-01774-8. Epub 2019 Sep 10. Mol Neurobiol. 2020. PMID: 31506900
-
Sex-chromosome complement and Activin-A shape the therapeutic potential of TNFR2 activation in a model of MS and CNP.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2426771122. doi: 10.1073/pnas.2426771122. Epub 2025 May 16. Proc Natl Acad Sci U S A. 2025. PMID: 40378000
References
-
- Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. Int Rev Neurobiol. 2007;79:377–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

