Development of the larval anterior neurogenic domains of Terebratalia transversa (Brachiopoda) provides insights into the diversification of larval apical organs and the spiralian nervous system
- PMID: 22273002
- PMCID: PMC3314550
- DOI: 10.1186/2041-9139-3-3
Development of the larval anterior neurogenic domains of Terebratalia transversa (Brachiopoda) provides insights into the diversification of larval apical organs and the spiralian nervous system
Abstract
Background: Larval features such as the apical organ, apical ciliary tuft, and ciliated bands often complicate the evaluation of hypotheses regarding the origin of the adult bilaterian nervous system. Understanding how neurogenic domains form within the bilaterian head and larval apical organ requires expression data from animals that exhibit aspects of both centralized and diffuse nervous systems at different life history stages. Here, we describe the expression of eight neural-related genes during the larval development of the brachiopod, Terebratalia transversa.
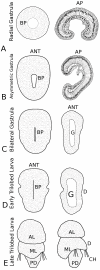
Results: Radially symmetric gastrulae broadly express Tt-Six3/6 and Tt-hbn in the animal cap ectoderm. Tt-NK2.1 and Tt-otp are restricted to a central subset of these cells, and Tt-fez and Tt-FoxQ2 expression domains are already asymmetric at this stage. As gastrulation proceeds, the spatial expression of these genes is split between two anterior ectodermal domains, a more dorsal region comprised of Tt-Six3/6, Tt-fez, Tt-FoxQ2, and Tt-otp expression domains, and an anterior ventral domain demarcated by Tt-hbn and Tt-NK2.1 expression. More posteriorly, the latter domains are bordered by Tt-FoxG expression in the region of the transverse ciliated band. Tt-synaptotagmin 1 is expressed throughout the anterior neural ectoderm. All genes are expressed late into larval development. The basiepithelial larval nervous system includes three neurogenic domains comprised of the more dorsal apical organ and a ventral cell cluster in the apical lobe as well as a mid-ventral band of neurons in the mantle lobe. Tt-otp is the only gene expressed in numerous flask-shaped cells of the apical organ and in a subset of neurons in the mantle lobe.
Conclusions: Our expression data for Tt-Six3/6, Tt-FoxQ2, and Tt-otp confirm some aspects of bilaterian-wide conservation of spatial partitioning within anterior neurogenic domains and also suggest a common origin for central otp-positive cell types within the larval apical organs of spiralians. However, the field of sensory neurons within the larval apical organ of Terebratalia is broader and composed of more cells relative to those of other spiralian larvae. These cellular differences are mirrored in the broader spatial and temporal expression patterns of Tt-FoxQ2 and Tt-otp. Corresponding differences in the expression of Tt-hbn, Tt-NK2.1, and Tt-FoxG are also observed relative to their respective domains within the cerebral ganglia of spiralians. Based on these data we argue that the anterior region of the bilaterian stem species included Six3/6, NK2.1, otp, hbn, fez, and FoxQ2 expression domains that were subsequently modified within larval and adult neural tissues of protostome and deuterostome animals.
Figures




Similar articles
-
Mesodermal gene expression during the embryonic and larval development of the articulate brachiopod Terebratalia transversa.Evodevo. 2015 Apr 11;6:10. doi: 10.1186/s13227-015-0004-8. eCollection 2015. Evodevo. 2015. PMID: 25897375 Free PMC article.
-
An anterior medial cell population with an apical-organ-like transcriptional profile that pioneers the central nervous system in the centipede Strigamia maritima.Dev Biol. 2014 Dec 1;396(1):136-49. doi: 10.1016/j.ydbio.2014.09.020. Epub 2014 Sep 26. Dev Biol. 2014. PMID: 25263198
-
Larval body patterning and apical organs are conserved in animal evolution.BMC Biol. 2014 Jan 29;12:7. doi: 10.1186/1741-7007-12-7. BMC Biol. 2014. PMID: 24476105 Free PMC article.
-
Larval nervous systems: true larval and precocious adult.J Exp Biol. 2015 Feb 15;218(Pt 4):629-36. doi: 10.1242/jeb.109603. J Exp Biol. 2015. PMID: 25696826 Review.
-
Embryonic neurogenesis in echinoderms.Wiley Interdiscip Rev Dev Biol. 2018 Jul;7(4):e316. doi: 10.1002/wdev.316. Epub 2018 Feb 22. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29470839 Review.
Cited by
-
Expression of segment polarity genes in brachiopods supports a non-segmental ancestral role of engrailed for bilaterians.Sci Rep. 2016 Aug 26;6:32387. doi: 10.1038/srep32387. Sci Rep. 2016. PMID: 27561213 Free PMC article.
-
A comprehensive study of arthropod and onychophoran Fox gene expression patterns.PLoS One. 2022 Jul 8;17(7):e0270790. doi: 10.1371/journal.pone.0270790. eCollection 2022. PLoS One. 2022. PMID: 35802758 Free PMC article.
-
The brain regulatory program predates central nervous system evolution.Sci Rep. 2023 May 27;13(1):8626. doi: 10.1038/s41598-023-35721-4. Sci Rep. 2023. PMID: 37244953 Free PMC article.
-
Conserved MIP receptor-ligand pair regulates Platynereis larval settlement.Proc Natl Acad Sci U S A. 2013 May 14;110(20):8224-9. doi: 10.1073/pnas.1220285110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569279 Free PMC article.
-
Nervous system development in lecithotrophic larval and juvenile stages of the annelid Capitella teleta.Front Zool. 2015 Jul 11;12:15. doi: 10.1186/s12983-015-0108-y. eCollection 2015. Front Zool. 2015. PMID: 26167198 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

