Transcriptional signature of accessory cells in the lateral line, using the Tnk1bp1:EGFP transgenic zebrafish line
- PMID: 22273551
- PMCID: PMC3305402
- DOI: 10.1186/1471-213X-12-6
Transcriptional signature of accessory cells in the lateral line, using the Tnk1bp1:EGFP transgenic zebrafish line
Abstract
Background: Because of the structural and molecular similarities between the two systems, the lateral line, a fish and amphibian specific sensory organ, has been widely used in zebrafish as a model to study the development/biology of neuroepithelia of the inner ear. Both organs have hair cells, which are the mechanoreceptor cells, and supporting cells providing other functions to the epithelium. In most vertebrates (excluding mammals), supporting cells comprise a pool of progenitors that replace damaged or dead hair cells. However, the lack of regenerative capacity in mammals is the single leading cause for acquired hearing disorders in humans.
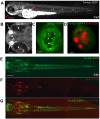
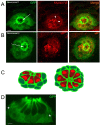
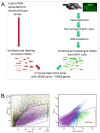
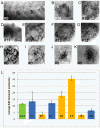
Results: In an effort to understand the regenerative process of hair cells in fish, we characterized and cloned an egfp transgenic stable fish line that trapped tnks1bp1, a highly conserved gene that has been implicated in the maintenance of telomeres' length. We then used this Tg(tnks1bp1:EGFP) line in a FACsorting strategy combined with microarrays to identify new molecular markers for supporting cells.
Conclusions: We present a Tg(tnks1bp1:EGFP) stable transgenic line, which we used to establish a transcriptional profile of supporting cells in the zebrafish lateral line. Therefore we are providing a new set of markers specific for supporting cells as well as candidates for functional analysis of this important cell type. This will prove to be a valuable tool for the study of regeneration in the lateral line of zebrafish in particular and for regeneration of neuroepithelia in general.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

