Synthesis and evaluation of 17α-(dimethylphenyl)vinyl estradiols as probes of the estrogen receptor-α ligand binding domain
- PMID: 22273809
- PMCID: PMC3307546
- DOI: 10.1016/j.steroids.2012.01.003
Synthesis and evaluation of 17α-(dimethylphenyl)vinyl estradiols as probes of the estrogen receptor-α ligand binding domain
Abstract
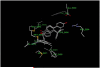
As part of our program to explore the influence of small structural modifications on the biological response of the estrogen receptor-α (ERα), we prepared and evaluated a series of mono-and di-substituted phenyl vinyl estradiols. The target compounds were prepared in 45-80% yields using the Stille coupling reaction and evaluated using competitive binding analysis with the ERα-ligand binding domain (hERα-LBD) and estrogenic activity (induction of alkaline phosphatase in Ishikawa cells). Results indicated that the 2,4- and 2,5-dimethyl derivatives, 5b and 5c, had the highest relative binding affinity (RBA=20.5 and 37.3%) and relative stimulatory activity (RSA=101.0% and 12.3%) of the di-methyl series.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
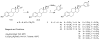
Similar articles
-
Synthesis of benzoylbenzamide derivatives of 17α-E-vinyl estradiol and evaluation as ligands for the estrogen receptor-α ligand binding domain.Steroids. 2019 Apr;144:15-20. doi: 10.1016/j.steroids.2019.02.003. Epub 2019 Feb 6. Steroids. 2019. PMID: 30738075
-
Synthesis and evaluation of 17α-E-20-(heteroaryl)norpregn-1,3,5(10),20 tetraene-3,17β-diols [17α-(heteroaryl)vinyl estradiols] as ligands for the estrogen receptor-α ligand binding domain (ERα-LBD).Bioorg Med Chem Lett. 2012 Jan 15;22(2):977-9. doi: 10.1016/j.bmcl.2011.12.003. Epub 2011 Dec 8. Bioorg Med Chem Lett. 2012. PMID: 22178552 Free PMC article.
-
Synthesis and evaluation of 17alpha-20E-21-(4-substituted phenyl)-19-norpregna-1,3,5(10),20-tetraene-3,17beta-diols as probes for the estrogen receptor alpha hormone binding domain.J Med Chem. 2003 Jul 3;46(14):2865-76. doi: 10.1021/jm0205806. J Med Chem. 2003. PMID: 12825929
-
Evaluation of 17alpha-E-(trifluoromethylphenyl)vinyl estradiols as novel estrogen receptor ligands.Steroids. 2003 Feb;68(2):143-8. doi: 10.1016/s0039-128x(02)00165-4. Steroids. 2003. PMID: 12606005
-
CoMSIA and docking study of rhenium based estrogen receptor ligand analogs.Steroids. 2007 Mar;72(3):247-60. doi: 10.1016/j.steroids.2006.11.011. Epub 2007 Feb 5. Steroids. 2007. PMID: 17280694 Free PMC article. Review.
Cited by
-
Application of Various Molecular Modelling Methods in the Study of Estrogens and Xenoestrogens.Int J Mol Sci. 2020 Sep 3;21(17):6411. doi: 10.3390/ijms21176411. Int J Mol Sci. 2020. PMID: 32899216 Free PMC article. Review.
-
Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer.J Med Chem. 2013 Jun 27;56(12):4880-98. doi: 10.1021/jm400048v. Epub 2013 Jun 7. J Med Chem. 2013. PMID: 23713567 Free PMC article.
-
Synthesis and evaluation of 11β-(4-substituted phenyl) estradiol analogs: transition from estrogen receptor agonists to antagonists.Bioorg Med Chem. 2012 Jun 15;20(12):3768-80. doi: 10.1016/j.bmc.2012.04.041. Epub 2012 May 7. Bioorg Med Chem. 2012. PMID: 22608920 Free PMC article.
References
-
- Napolitano E, Fiaschi R, Herman LW, Hanson RN. Steroids. 1996;61:384. - PubMed
-
- Hanson RN, Herman LW, Fiaschi R, Napolitano E. Steroids. 1996;61:718. - PubMed
-
- Hanson RN, Napolitano E, Fiaschi R. Steroids. 1998;63:479. - PubMed
-
- Hanson R, Napolitano E, Fiaschi R. J. Med. Chem. 1998;41:4686. - PubMed
-
- Hanson RN, Lee CY, Friel CJ, Dilis R, Hughes A, DeSombre ER. J. Med. Chem. 2003;46:2865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

