Assessing the contribution of cell body and intracellular organelles to the backward light scattering
- PMID: 22274427
- PMCID: PMC3340331
- DOI: 10.1364/OE.20.000816
Assessing the contribution of cell body and intracellular organelles to the backward light scattering
Abstract
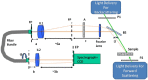
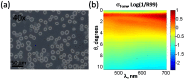
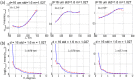
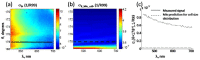
We report a method of assessing the contribution of whole cell body and its nucleus to the clinically most relevant backward light scattering. We first construct an experimental system that can measure forward scattering and use the system to precisely extract the optical properties of a specimen such as the refractive index contrast, size distribution, and their density. A system that can simultaneously detect the backscattered light is installed to collect the backscattering for the same specimen. By comparing the measured backscattering spectrum with that estimated from the parameters determined by the forward scattering experiment, the contribution of cell body and nucleus to the backward light scattering is quantitatively assessed. For the HeLa cells in suspension, we found that the cell body contributes less than 10% and cell nucleus on the order of 0.1% to the total backscattering signal. Quantitative determination of the origin of backscattered light may help design a system that aims for detecting particular structure of biological tissues.
Figures


References
-
- Backman V., Gurjar R., Badizadegan K., Itzkan L., Dasari R. R., Perelman L. T., Feld M. S., “Polarized light scattering spectroscopy for quantitative measurement of epithelial cellular structures in situ,” IEEE J. Sel. Top. Quantum Electron. 5(4), 1019–1026 (1999).10.1109/2944.796325 - DOI
-
- Mujat C., Greiner C., Baldwin A., Levitt J. M., Tian F., Stucenski L. A., Hunter M., Kim Y. L., Backman V., Feld M., Münger K., Georgakoudi I., “Endogenous optical biomarkers of normal and human papillomavirus immortalized epithelial cells,” Int. J. Cancer 122(2), 363–371 (2008).10.1002/ijc.23120 - DOI - PubMed
-
- Fang H., Ollero M., Vitkin E., Kimerer L. M., Cipolloni P. B., Zaman M. M., Freedman S. D., Bigio I. J., Itzkan I., Hanlon E. B., Perelman L. T., “Noninvasive sizing of subcellular organelles with light scattering spectroscopy,” IEEE J. Sel. Top. Quantum Electron. 9(2), 267–276 (2003).10.1109/JSTQE.2003.812515 - DOI
-
- Hunter M., Backman V., Popescu G., Kalashnikov M., Boone C. W., Wax A., Gopal V., Badizadegan K., Stoner G. D., Feld M. S., “Tissue self-affinity and polarized light scattering in the born approximation: A new model for precancer detection,” Phys. Rev. Lett. 97(13), 138102 (2006).10.1103/PhysRevLett.97.138102 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

