Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis
- PMID: 22274627
- PMCID: PMC3289569
- DOI: 10.1105/tpc.111.090597
Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis
Abstract
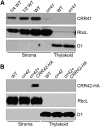
Chloroplast NADH dehydrogenase-like complex (NDH) mediates photosystem I cyclic electron transport and chlororespiration in thylakoids. Recently, substantial progress has been made in understanding the structure of NDH, but our knowledge of its assembly has been limited. In this study, a series of interactive proteomic analyses identified several stroma-localized factors required for the assembly of a stroma-protruding arm of NDH (subcomplex A). In addition to further characterization of the previously identified CHLORORESPIRATORY REDUCTION1 (CRR1), CRR6, and CRR7, two novel stromal proteins, CRR41 and CRR42, were discovered. Arabidopsis thaliana mutants lacking these proteins are specifically defective in the accumulation of subcomplex A. A total of 10 mutants lacking subcomplex A, including crr27/cpn60β4, which is specifically defective in the folding of NdhH, and four mutants lacking NdhL-NdhO subunits, were extensively characterized. We propose a model for subcomplex A assembly: CRR41, NdhO, and native NdhH, as well as unknown factors, are first assembled to form an NDH subcomplex A assembly intermediate (NAI500). Subsequently, NdhJ, NdhM, NdhK, and NdhI are incorporated into NAI500 to form NAI400. CRR1, CRR6, and CRR42 are involved in this process. CRR7 is likely to be involved in the final step, in which the fully assembled NAI, including NdhN, is inserted into thylakoids.
Figures




Similar articles
-
Chloroplast stromal proteins, CRR6 and CRR7, are required for assembly of the NAD(P)H dehydrogenase subcomplex A in Arabidopsis.Plant J. 2010 Jul;63(2):203-211. doi: 10.1111/j.1365-313X.2010.04240.x. Epub 2010 Apr 28. Plant J. 2010. Retraction in: Plant J. 2023 Apr;114(2):455. doi: 10.1111/tpj.16170. PMID: 20444231 Retracted.
-
CHLORORESPIRATORY REDUCTION 9 is a Novel Factor Required for Formation of Subcomplex A of the Chloroplast NADH Dehydrogenase-Like Complex.Plant Cell Physiol. 2016 Oct;57(10):2122-2132. doi: 10.1093/pcp/pcw130. Epub 2016 Aug 1. Plant Cell Physiol. 2016. PMID: 27481895
-
The NdhV subunit is required to stabilize the chloroplast NADH dehydrogenase-like complex in Arabidopsis.Plant J. 2015 Apr;82(2):221-31. doi: 10.1111/tpj.12807. Epub 2015 Mar 18. Plant J. 2015. PMID: 25728844
-
Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits.Plant Cell Physiol. 2011 Sep;52(9):1560-8. doi: 10.1093/pcp/pcr098. Epub 2011 Jul 23. Plant Cell Physiol. 2011. PMID: 21785130 Review.
-
Towards characterization of the chloroplast NAD(P)H dehydrogenase complex.Mol Plant. 2009 Nov;2(6):1127-40. doi: 10.1093/mp/ssp052. Epub 2009 Jul 21. Mol Plant. 2009. PMID: 19995722 Review.
Cited by
-
Visualizing structural dynamics of thylakoid membranes.Sci Rep. 2014 Jan 20;4:3768. doi: 10.1038/srep03768. Sci Rep. 2014. PMID: 24442007 Free PMC article.
-
NdhP is an exclusive subunit of large complex of NADPH dehydrogenase essential to stabilize the complex in Synechocystis sp. strain PCC 6803.J Biol Chem. 2014 Jul 4;289(27):18770-81. doi: 10.1074/jbc.M114.553404. Epub 2014 May 20. J Biol Chem. 2014. PMID: 24847053 Free PMC article.
-
NdhM Subunit Is Required for the Stability and the Function of NAD(P)H Dehydrogenase Complexes Involved in CO2 Uptake in Synechocystis sp. Strain PCC 6803.J Biol Chem. 2016 Mar 11;291(11):5902-5912. doi: 10.1074/jbc.M115.698084. Epub 2015 Dec 24. J Biol Chem. 2016. PMID: 26703473 Free PMC article.
-
NdhV Is a Subunit of NADPH Dehydrogenase Essential for Cyclic Electron Transport in Synechocystis sp. Strain PCC 6803.Plant Physiol. 2016 Feb;170(2):752-60. doi: 10.1104/pp.15.01430. Epub 2015 Dec 7. Plant Physiol. 2016. PMID: 26644505 Free PMC article.
-
Subunit Q Is Required to Stabilize the Large Complex of NADPH Dehydrogenase in Synechocystis sp. Strain PCC 6803.Plant Physiol. 2015 Jun;168(2):443-51. doi: 10.1104/pp.15.00503. Epub 2015 Apr 14. Plant Physiol. 2015. PMID: 25873552 Free PMC article.
References
-
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 - PubMed
-
- Efremov R.G., Baradaran R., Sazanov L.A. (2010). The architecture of respiratory complex I. Nature 465: 441–445 - PubMed
-
- Efremov R.G., Sazanov L.A. (2011). Structure of the membrane domain of respiratory complex I. Nature 476: 414–420 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

