Lansoprazole for children with poorly controlled asthma: a randomized controlled trial
- PMID: 22274684
- PMCID: PMC4153372
- DOI: 10.1001/jama.2011.2035
Lansoprazole for children with poorly controlled asthma: a randomized controlled trial
Abstract
Context: Asymptomatic gastroesophageal reflux (GER) is prevalent in children with asthma. Untreated GER has been postulated to be a cause of inadequate asthma control in children despite inhaled corticosteroid treatment, but it is not known whether treatment with proton pump inhibitors improves asthma control.
Objective: To determine whether lansoprazole is effective in reducing asthma symptoms in children without overt GER.
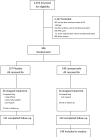
Design, setting, and participants: The Study of Acid Reflux in Children With Asthma, a randomized, masked, placebo-controlled, parallel clinical trial that compared lansoprazole with placebo in children with poor asthma control who were receiving inhaled corticosteroid treatment. Three hundred six participants enrolled from April 2007 to September 2010 at 19 US academic clinical centers were followed up for 24 weeks. A subgroup had an esophageal pH study before randomization.
Intervention: Participating children were randomly assigned to receive either lansoprazole, 15 mg/d if weighing less than 30 kg or 30 mg/d if weighing 30 kg or more (n = 149), or placebo (n = 157).
Main outcome measures: The primary outcome measure was change in Asthma Control Questionnaire (ACQ) score (range, 0-6; a 0.5-unit change is considered clinically meaningful). Secondary outcome measures included lung function measures, asthma-related quality of life, and episodes of poor asthma control.
Results: The mean age was 11 years (SD, 3 years). The mean difference in change (lansoprazole minus placebo) in the ACQ score was 0.2 units (95% CI, 0.0-0.3 units). There were no statistically significant differences in the mean difference in change for the secondary outcomes of forced expiratory volume in the first second (0.0 L; 95% CI, -0.1 to 0.1 L), asthma-related quality of life (-0.1; 95% CI, -0.3 to 0.1), or rate of episodes of poor asthma control (relative risk, 1.2; 95% CI, 0.9-1.5). Among the 115 children with esophageal pH studies, the prevalence of GER was 43%. In the subgroup with a positive pH study, no treatment effect for lansoprazole vs placebo was observed for any asthma outcome. Children treated with lansoprazole reported more respiratory infections (relative risk, 1.3 [95% CI, 1.1-1.6]).
Conclusion: In this trial of children with poorly controlled asthma without symptoms of GER who were using inhaled corticosteroids, the addition of lansoprazole, compared with placebo, improved neither symptoms nor lung function but was associated with increased adverse events.
Trial registration: clinicaltrials.gov Identifier: NCT00442013.
Figures
Comment in
-
Children, asthma, and proton pump inhibitors: costs and perils of therapeutic creep.JAMA. 2012 Jan 25;307(4):406-7. doi: 10.1001/jama.2012.33. JAMA. 2012. PMID: 22274689 No abstract available.
-
Lansoprazole of no benefit in children with asthma.J Pediatr. 2012 Jul;161(1):170. doi: 10.1016/j.jpeds.2012.04.051. J Pediatr. 2012. PMID: 22726330 No abstract available.
-
Lansoprazole for children with poorly controlled asthma.Arch Dis Child Educ Pract Ed. 2013 Oct;98(5):199. doi: 10.1136/archdischild-2013-304692. Epub 2013 Aug 21. Arch Dis Child Educ Pract Ed. 2013. PMID: 23966207 No abstract available.
References
-
- Tucci F, Resti M, Fontana R, Novembre E, Lami CA, Vierucci A. Gastroesophageal reflux and bronchial asthma: prevalence and effect of cisapride therapy. J Pediatr Gastroenterol Nutr. 1993 Oct;17(3):265–70. - PubMed
-
- Cinquetti M, Micelli S, Voltolina C, Zoppi G. The pattern of gastroesophageal reflux in asthmatic children. J Asthma. 2002 Apr;39(2):135–42. - PubMed
-
- Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009 Oct;49(4):498–547. - PubMed
-
- Andze GO, Brandt ML, St Vil D, Bensoussan AL, Blanchard H. Diagnosis and treatment of gastroesophageal reflux in 500 children with respiratory symptoms: The value of pH monitoring. J Pediatr Surg. 1991 Mar;26(3):295–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous