Myoblast fusion: lessons from flies and mice
- PMID: 22274696
- PMCID: PMC3265056
- DOI: 10.1242/dev.068353
Myoblast fusion: lessons from flies and mice
Abstract
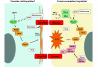
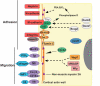
The fusion of myoblasts into multinucleate syncytia plays a fundamental role in muscle function, as it supports the formation of extended sarcomeric arrays, or myofibrils, within a large volume of cytoplasm. Principles learned from the study of myoblast fusion not only enhance our understanding of myogenesis, but also contribute to our perspectives on membrane fusion and cell-cell fusion in a wide array of model organisms and experimental systems. Recent studies have advanced our views of the cell biological processes and crucial proteins that drive myoblast fusion. Here, we provide an overview of myoblast fusion in three model systems that have contributed much to our understanding of these events: the Drosophila embryo; developing and regenerating mouse muscle; and cultured rodent muscle cells.
Figures





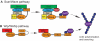
References
-
- Abramovici H., Gee S. H. (2007). Morphological changes and spatial regulation of diacylglycerol kinase-zeta, syntrophins, and Rac1 during myoblast fusion. Cell Motil. Cytoskel. 64, 549–567 - PubMed
-
- Anton I. M., Jones G. E., Wandosell F., Geha R., Ramesh N. (2007). WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell Biol. 17, 555–562 - PubMed
-
- Artero R. D., Castanon I., Baylies M. K. (2001). The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128, 4251–4264 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

