Identification and mechanistic studies of a novel ubiquitin E1 inhibitor
- PMID: 22274912
- PMCID: PMC3339042
- DOI: 10.1177/1087057111433843
Identification and mechanistic studies of a novel ubiquitin E1 inhibitor
Abstract
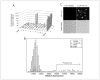
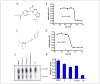
Protein degradation via the ubiquitin-proteasome pathway is important for a diverse number of cellular processes ranging from cell signaling to development. Disruption of the ubiquitin pathway occurs in a variety of human diseases, including several cancers and neurological disorders. Excessive proteolysis of tumor suppressor proteins, such as p27, occurs in numerous aggressive human tumors. To discover small-molecule inhibitors that potentially prevent p27 degradation, we developed a series of screening assays, including a cell-based screen of a small-molecule compound library and two novel nucleotide exchange assays. Several small-molecule inhibitors, including NSC624206, were identified and subsequently verified to prevent p27 ubiquitination in vitro. The mechanism of NSC624206 inhibition of p27 ubiquitination was further unraveled using the nucleotide exchange assays and shown to be due to antagonizing ubiquitin activating enzyme (E1). We determined that NSC624206 and PYR-41, a recently reported inhibitor of ubiquitin E1, specifically block ubiquitin-thioester formation but have no effect on ubiquitin adenylation. These studies reveal a novel E1 inhibitor that targets a specific step of the E1 activation reaction. NSC624206 could, therefore, be potentially useful for the control of excessive ubiquitin-mediated proteolysis in vivo.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
-
- Hershko A, Ciechanover A. Mechanisms of Intracellular Protein Breakdown. Annu Rev Biochem. 1982;51:335–364. - PubMed
-
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist. 2003;8:508–513. - PubMed
-
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib Plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–917. - PubMed
-
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the Ubiquitin-Proteasome Pathway in Regulating Abundance of the Cyclin-Dependent Kinase Inhibitor p27. Science. 1995;269:682–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

