Rationally designed oxaliplatin-nanoparticle for enhanced antitumor efficacy
- PMID: 22275055
- PMCID: PMC3387556
- DOI: 10.1088/0957-4484/23/7/075103
Rationally designed oxaliplatin-nanoparticle for enhanced antitumor efficacy
Abstract
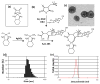
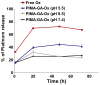
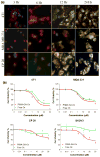
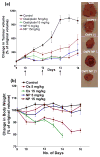
Nanoscale drug delivery vehicles have been extensively studied as carriers for cancer chemotherapeutics. However, the formulation of platinum chemotherapeutics in nanoparticles has been a challenge arising from their physicochemical properties. There are only a few reports describing oxaliplatin nanoparticles. In this study, we derivatized the monomeric units of a polyisobutylene maleic acid copolymer with glucosamine, which chelates trans-1,2-diaminocyclohexane (DACH) platinum (II) through a novel monocarboxylato and O --> Pt coordination linkage. At a specific polymer to platinum ratio, the complex self-assembled into a nanoparticle, where the polymeric units act as the leaving group, releasing DACH-platinum in a sustained pH-dependent manner. Sizing was done using dynamic light scatter and electron microscopy. The nanoparticles were evaluated for efficacy in vitro and in vivo. Biodistribution was quantified using inductively coupled plasma atomic absorption spectroscopy (ICP-AAS). The PIMA-GA-DACH-platinum nanoparticle was found to be more active than free oxaliplatin in vitro. In vivo, the nanoparticles resulted in greater tumor inhibition than oxaliplatin (equivalent to 5 mg kg⁻¹ platinum dose) with minimal nephrotoxicity or body weight loss. ICP-AAS revealed significant preferential tumor accumulation of platinum with reduced biodistribution to the kidney or liver following PIMA-GA-DACH-platinum nanoparticle administration as compared with free oxaliplatin. These results indicate that the rational engineering of a novel polymeric nanoparticle inspired by the bioactivation of oxaliplatin results in increased antitumor potency with reduced systemic toxicity compared with the parent cytotoxic. Rational design can emerge as an exciting strategy in the synthesis of nanomedicines for cancer chemotherapy.
Figures


References
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nature Rev Cancer. 2007;7:573–584. - PubMed
-
- Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med. 1978;65:307–14. - PubMed
-
- Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato) platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–9. - PubMed
-
- Kasparová D, Vrána O, Kleinwächter V, Brabec V. The effect of platinum derivatives on interfacial properties of DNA. Biophys Chem. 1987;28:191–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
