Association of industry funding with the outcome and quality of randomized controlled trials of drug therapy for rheumatoid arthritis
- PMID: 22275179
- PMCID: PMC3656717
- DOI: 10.1002/art.34393
Association of industry funding with the outcome and quality of randomized controlled trials of drug therapy for rheumatoid arthritis
Abstract
Objective: To assess the association of industry funding with the characteristics, outcome, and reported quality of randomized controlled trials (RCTs) of drug therapy for rheumatoid arthritis (RA).
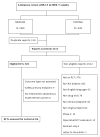
Methods: The Medline and Cochrane Central Register of Controlled Trials databases were searched to identify original RA drug therapy RCTs published in 2002-2003 and 2006-2007. Two reviewers independently assessed each RCT for the funding source, characteristics, outcome (positive [statistically significant result favoring experimental drug for the primary outcome] or not positive), and reporting of methodologic measures whose inadequate performance may have biased the assessment of treatment effect. RCTs that were registered at ClinicalTrials.gov and completed during the study years were assessed for publication bias.
Results: Of the 103 eligible RCTs identified, 58 (56.3%) were funded by industry, 19 (18.4%) were funded by nonprofit sources, 6 (5.8%) had mixed funding, and funding for 20 (19.4%) was not specified. Industry-funded RCTs had significantly more study centers and subjects, while nonprofit agency-funded RCTs had longer duration and were more likely to study different treatment strategies. Outcome could be assessed for 86 (83.5%) of the 103 RCTs studied. The funding source was not associated with a higher likelihood of positive outcomes favoring the sponsored experimental drug (75.5% of industry-funded RCTs had a positive outcome, compared with 68.8% of non-industry-funded RCTs, 40% of RCTs with mixed funding, and 81.2% of RCTs for which funding was not specified). Industry-funded RCTs showed a trend toward a higher likelihood of nonpublication (P=0.093). Industry-funded RCTs were more frequently associated with double-blinding, an adequate description of participant flow, and performance of an intent-to-treat analysis.
Conclusion: Industry funding was not associated with a higher likelihood of positive outcomes of published RCTs of drug therapy for RA, and industry-funded RCTs performed significantly better than non-industry-funded RCTs in terms of reporting the use of some key methodologic quality measures.
Copyright © 2012 by the American College of Rheumatology.
Conflict of interest statement
None of the authors have any financial conflict of interest relevant to the manuscript.
Figures
References
-
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Ann Pharmacother. 2004;38:579–85. - PubMed
-
- Fontanarosa PB, Flanagin A, DeAngelis CD. Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies. JAMA. 2005;294:110–1. - PubMed
-
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical