The RON-receptor regulates pancreatic cancer cell migration through phosphorylation-dependent breakdown of the hemidesmosome
- PMID: 22275185
- PMCID: PMC3424378
- DOI: 10.1002/ijc.27447
The RON-receptor regulates pancreatic cancer cell migration through phosphorylation-dependent breakdown of the hemidesmosome
Abstract
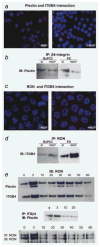
The recepteur d'origine nantais (RON) receptor tyrosine kinase is overexpressed and stimulates invasive growth in pancreatic cancer cells, yet the mechanisms that underlie RON-mediated phenotypes remain poorly characterized. To better understand RON function in pancreatic cancer cells, we sought to identify novel RON interactants using multidimensional protein identification analysis. These studies revealed plectin, a large protein of the spectrin superfamily, to be a novel RON interactant. Plectin is a multifunctional protein that complexes with integrin-β4 (ITGB4) to form hemidesmosomes, serves as a scaffolding platform crucial to the function of numerous protein signaling pathways and was recently described as an overexpressed protein in pancreatic cancer (Bausch D et al., Clin Cancer Res 2010; Kelly et al., PLoS Med 2008;5:e85). In this study, we demonstrate that on exposure to its ligand, macrophage-stimulating protein, RON binds to plectin and ITGB4, which results in disruption of the plectin-ITGB4 interaction and enhanced cell migration, a phenotype that can be recapitulated by small hairpin ribosomal nucleic acid (shRNA)-mediated suppression of plectin expression. We demonstrate that disruption of plectin-ITGB4 is dependent on RON and phosphoinositide-3 (PI3) kinase, but not mitogen-activated protein kinase (MEK), activity. Thus, in pancreatic cancer cells, plectin and ITGB4 form hemidesmosomes which serve to anchor cells to the extracellular matrix (ECM) and restrain migration. The current study defines a novel interaction between RON and plectin, provides new insight into RON-mediated migration and further supports efforts to target RON kinase activity in pancreatic cancer.
Copyright © 2012 UICC.
Figures



Similar articles
-
IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration.Carcinogenesis. 2011 Aug;32(8):1151-6. doi: 10.1093/carcin/bgr086. Epub 2011 May 11. Carcinogenesis. 2011. PMID: 21565828 Free PMC article.
-
Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly.Mol Biol Cell. 2012 Apr;23(8):1475-85. doi: 10.1091/mbc.E11-11-0957. Epub 2012 Feb 22. Mol Biol Cell. 2012. PMID: 22357621 Free PMC article.
-
Ron knockdown and Ron monoclonal antibody IMC-RON8 sensitize pancreatic cancer to histone deacetylase inhibitors (HDACi).PLoS One. 2013 Jul 29;8(7):e69992. doi: 10.1371/journal.pone.0069992. Print 2013. PLoS One. 2013. PMID: 23922886 Free PMC article.
-
Current insights into the formation and breakdown of hemidesmosomes.Trends Cell Biol. 2006 Jul;16(7):376-83. doi: 10.1016/j.tcb.2006.05.004. Epub 2006 Jun 6. Trends Cell Biol. 2006. PMID: 16757171 Review.
-
The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies.Pancreas. 2014 Mar;43(2):183-9. doi: 10.1097/MPA.0000000000000088. Pancreas. 2014. PMID: 24518495 Free PMC article. Review.
Cited by
-
Junctional epithelium and hemidesmosomes: Tape and rivets for solving the "percutaneous device dilemma" in dental and other permanent implants.Bioact Mater. 2022 Mar 19;18:178-198. doi: 10.1016/j.bioactmat.2022.03.019. eCollection 2022 Dec. Bioact Mater. 2022. PMID: 35387164 Free PMC article. Review.
-
Therapeutic evaluation of monoclonal antibody-maytansinoid conjugate as a model of RON-targeted drug delivery for pancreatic cancer treatment.Am J Cancer Res. 2016 May 1;6(5):937-56. eCollection 2016. Am J Cancer Res. 2016. PMID: 27293990 Free PMC article.
-
Ping-Pong-Tumor and Host in Pancreatic Cancer Progression.Front Oncol. 2019 Dec 16;9:1359. doi: 10.3389/fonc.2019.01359. eCollection 2019. Front Oncol. 2019. PMID: 31921628 Free PMC article. Review.
-
Integrin α6β4 Upregulates Amphiregulin and Epiregulin through Base Excision Repair-Mediated DNA Demethylation and Promotes Genome-wide DNA Hypomethylation.Sci Rep. 2017 Jul 21;7(1):6174. doi: 10.1038/s41598-017-06351-4. Sci Rep. 2017. PMID: 28733611 Free PMC article.
-
MicroRNA-375 Functions as a Tumor-Suppressor Gene in Gastric Cancer by Targeting Recepteur d'Origine Nantais.Int J Mol Sci. 2016 Sep 27;17(10):1633. doi: 10.3390/ijms17101633. Int J Mol Sci. 2016. PMID: 27689991 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE, Lowy AM. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–82. - PubMed
-
- Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–203. - PubMed
-
- Leung CL, Green KJ, Liem RK. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 2002;12:37–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

