Retinal imaging and image analysis
- PMID: 22275207
- PMCID: PMC3131209
- DOI: 10.1109/RBME.2010.2084567
Retinal imaging and image analysis
Abstract
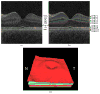
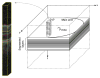
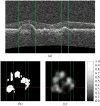
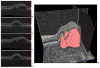
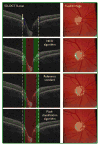
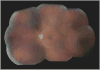
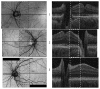
Many important eye diseases as well as systemic diseases manifest themselves in the retina. While a number of other anatomical structures contribute to the process of vision, this review focuses on retinal imaging and image analysis. Following a brief overview of the most prevalent causes of blindness in the industrialized world that includes age-related macular degeneration, diabetic retinopathy, and glaucoma, the review is devoted to retinal imaging and image analysis methods and their clinical implications. Methods for 2-D fundus imaging and techniques for 3-D optical coherence tomography (OCT) imaging are reviewed. Special attention is given to quantitative techniques for analysis of fundus photographs with a focus on clinically relevant assessment of retinal vasculature, identification of retinal lesions, assessment of optic nerve head (ONH) shape, building retinal atlases, and to automated methods for population screening for retinal diseases. A separate section is devoted to 3-D analysis of OCT images, describing methods for segmentation and analysis of retinal layers, retinal vasculature, and 2-D/3-D detection of symptomatic exudate-associated derangements, as well as to OCT-based analysis of ONH morphology and shape. Throughout the paper, aspects of image acquisition, image analysis, and clinical relevance are treated together considering their mutually interlinked relationships.
Figures



















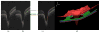




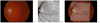

References
-
- Van Trigt AC. Trajecti ad Rhenum. 1853. Dissertatio ophthalmologica inauguralis de speculo oculi.
-
- Kolb H. How the retina works. Amer Scientist. 2003;91(1):28–35.
-
- Kolb H, Fernandez E, Nelson R, Jones BW. Webvision: Organization of the retina and visual system. 2005 [Online]. Available: http://webvision.med.utah.edu/ - PubMed
-
- WHO. [Online]. Available: http://www.who.int/diabetes/publications/Definitionanddiagnosisofdiabete....
-
- Nat. Eye Inst. Visual problems in the U.S. Nat Eye Inst. 2002 [Online]. Available: http://www.nei.nih.gov/eyedata/pdf/VPUS.pdf.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

