Notch pathway regulation of neural crest cell development in vivo
- PMID: 22275227
- PMCID: PMC3266628
- DOI: 10.1002/dvdy.23717
Notch pathway regulation of neural crest cell development in vivo
Abstract
Background: The function of Notch signaling in murine neural crest-derived cell lineages in vivo was examined.
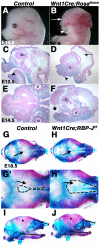
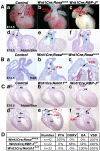
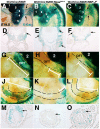
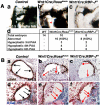
Results: Conditional gain (Wnt1Cre;Rosa(Notch)) or loss (Wnt1Cre;RBP-J(f/f)) of Notch signaling in neural crest cells (NCCs) in vivo results in craniofacial, cardiac, and trunk abnormalities. Severe craniofacial malformations are apparent in Wnt1Cre;Rosa(Notch) embryos, while less severe skull abnormalities are evident in Wnt1Cre;RBP-J(f/f) mice. Deficient cardiac neural crest migration, resulting in cardiac outflow tract malformations, occurs with increased or decreased Notch signaling in NCCs. Smooth muscle cell differentiation also is impaired in pharyngeal NCC derivatives in both Wnt1Cre;Rosa(Notch) and Wnt1Cre;RBP-J(f/f) embryos. Neurogenesis is absent and gliogenesis is increased in the dorsal root ganglia of Wnt1Cre;Rosa(Notch) embryos, while neurogenesis is increased and gliogenesis is decreased in Wnt1Cre;RBP-J(f/f) embryos.
Conclusions: Together, these studies demonstrate essential cell-autonomous roles for appropriate levels of Notch signaling during NCC migration, proliferation, and differentiation with critical implications in craniofacial, cardiac, and neurogenic development and disease.
Copyright © 2012 Wiley-Liss, Inc.
Figures


Similar articles
-
Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells.Development. 2004 Jul;131(14):3481-90. doi: 10.1242/dev.01214. Development. 2004. PMID: 15226263
-
Focal adhesion kinase is required for neural crest cell morphogenesis during mouse cardiovascular development.J Clin Invest. 2009 Aug;119(8):2218-30. doi: 10.1172/JCI38194. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587446 Free PMC article.
-
Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice.Dev Biol. 2006 Jan 15;289(2):420-9. doi: 10.1016/j.ydbio.2005.11.008. Epub 2005 Dec 5. Dev Biol. 2006. PMID: 16332365
-
Neural crest cells in cardiovascular development.Curr Top Dev Biol. 2015;111:183-200. doi: 10.1016/bs.ctdb.2014.11.006. Epub 2015 Jan 20. Curr Top Dev Biol. 2015. PMID: 25662261 Review.
-
Neural crest contribution to the cardiovascular system.Adv Exp Med Biol. 2006;589:134-54. doi: 10.1007/978-0-387-46954-6_8. Adv Exp Med Biol. 2006. PMID: 17076279 Review.
Cited by
-
Molecular control of the neural crest and peripheral nervous system development.Curr Top Dev Biol. 2015;111:201-31. doi: 10.1016/bs.ctdb.2014.11.007. Epub 2015 Jan 22. Curr Top Dev Biol. 2015. PMID: 25662262 Free PMC article. Review.
-
Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives.Int J Mol Sci. 2018 Dec 13;19(12):4025. doi: 10.3390/ijms19124025. Int J Mol Sci. 2018. PMID: 30551562 Free PMC article.
-
Exome sequencing in multiplex families with left-sided cardiac defects has high yield for disease gene discovery.PLoS Genet. 2022 Jun 23;18(6):e1010236. doi: 10.1371/journal.pgen.1010236. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35737725 Free PMC article.
-
Notch controls the cell cycle to define leader versus follower identities during collective cell migration.Elife. 2022 Apr 19;11:e73550. doi: 10.7554/eLife.73550. Elife. 2022. PMID: 35438077 Free PMC article.
-
Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts.Basic Res Cardiol. 2014;109(5):431. doi: 10.1007/s00395-014-0431-z. Epub 2014 Aug 9. Basic Res Cardiol. 2014. PMID: 25107324 Free PMC article.
References
-
- Bergwerff M, DeRuiter MC, Hall S, Poelmann RE, Gittenberger-de Groot AC. Unique vascular morphology of the fourth aortic arches: possible implications for pathogenesis of type-B aortic arch interruption and anomalous right subclavian artery. Cardiovasc. Res. 1999;44:185–196. - PubMed
-
- Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr. Rev. 2007;28:339–363. - PubMed
-
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

