Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus
- PMID: 22275291
- PMCID: PMC3350827
- DOI: 10.1002/art.34400
Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus
Abstract
Objective: To assess the effects of the B lymphocyte stimulator (BLyS)-specific inhibitor belimumab on immunologic biomarkers, including B cell and T cell populations, and maintenance of antibody titers to prior vaccines in autoantibody-positive systemic lupus erythematosus (SLE) patients.
Methods: Pooled data from 2 phase III trials, the Study of Belimumab in Subjects with SLE 52-week (BLISS-52) and 76-week (BLISS-76) trials, comparing belimumab 1 mg/kg or 10 mg/kg versus placebo (plus standard SLE therapy for each group) were analyzed for changes in autoantibody, immunoglobulin, and complement levels. BLISS-76 patients were also analyzed for changes in B cell and T cell populations and effects on prior vaccine-induced antibody levels.
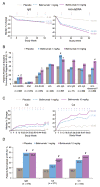
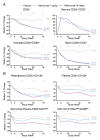
Results: Belimumab-treated patients experienced significant sustained reductions in IgG and autoantibodies and improvement in C3/C4 levels, resulting in greater positive-to-negative conversion rates for IgG anti-double-stranded DNA (anti-dsDNA), anti-Sm, anticardiolipin, and anti-ribosomal P autoantibodies and normalization of hypergammaglobulinemia and low C3/C4 levels. Belimumab-treated patients experienced significant decreases in the numbers of naive and activated B cells, as well as plasma cells, whereas memory B cells and T cell populations did not decrease. Belimumab did not substantially affect preexisting antipneumococcal or anti-tetanus toxoid antibody levels. Post hoc analysis showed greater reductions in SLE disease activity and the risk of severe flares in patients treated with belimumab 10 mg/kg (P≤0.01) who were anti-dsDNA positive and had low C3/C4 levels at baseline. Normalization of the C3 or anti-dsDNA level by 8 weeks, irrespective of therapy, was predictive of a reduced risk of severe flare over 52 weeks.
Conclusion: Belimumab appears to promote normalization of serologic activity and reduce BLyS-dependent B cell subsets in serologically and clinically active SLE. Greater serologic activity may predict a better treatment response to belimumab.
Trial registration: ClinicalTrials.gov NCT00410384 NCT00424476.
Copyright © 2012 by the American College of Rheumatology.
Conflict of interest statement
Dr Stohl has received clinical trials support from Human Genome Sciences (HGS). Dr Hiepe has received consultancy fees from HGS and GlaxoSmithKline (GSK). Dr Latinis has received fees for consultancy and speaker’s bureau participation from HGS and GSK. Dr Clarke has received research support and consultancy fees from HGS and GSK, and clinical trials support from HGS. Dr Aranow has received consultancy fees from HGS. Dr Wellborne has received research support from HGS, and consultancy fees from HGS and GSK. Drs Hough, Pineda, Migone, Zhong, and Freimuth are employed by and own stock in HGS. The other authors declare that they have no conflicts of interest.
Figures
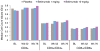
References
-
- Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol. 2009;5:400–4. - PubMed
-
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. - PubMed
-
- Arbuckle MR, McClain M, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. - PubMed
-
- Muñoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, et al. SLE—a disease of clearance deficiency? Rheumatology. 2005;44:1101–7. - PubMed
-
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

