Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis
- PMID: 22275356
- PMCID: PMC3322861
- DOI: 10.1074/jbc.M111.302547
Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis
Abstract
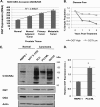
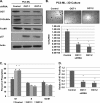
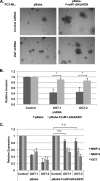
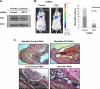
Cancer cells universally increase glucose and glutamine consumption, leading to the altered metabolic state known as the Warburg effect; one metabolic pathway, highly dependent on glucose and glutamine, is the hexosamine biosynthetic pathway. Increased flux through the hexosamine biosynthetic pathway leads to increases in the post-translational addition of O-linked β-N-acetylglucosamine (O-GlcNAc) to various nuclear and cytosolic proteins. A number of these target proteins are implicated in cancer, and recently, O-GlcNAcylation was shown to play a role in breast cancer; however, O-GlcNAcylation in other cancers remains poorly defined. Here, we show that O-GlcNAc transferase (OGT) is overexpressed in prostate cancer compared with normal prostate epithelium and that OGT protein and O-GlcNAc levels are elevated in prostate carcinoma cell lines. Reducing O-GlcNAcylation in PC3-ML cells was associated with reduced expression of matrix metalloproteinase (MMP)-2, MMP-9, and VEGF, resulting in inhibition of invasion and angiogenesis. OGT-mediated regulation of invasion and angiogenesis was dependent upon regulation of the oncogenic transcription factor FoxM1, a key regulator of invasion and angiogenesis, as reducing OGT expression led to increased FoxM1 protein degradation. Conversely, overexpression of a degradation-resistant FoxM1 mutant abrogated OGT RNAi-mediated effects on invasion, MMP levels, angiogenesis, and VEGF expression. Using a mouse model of metastasis, we found that reduction of OGT expression blocked bone metastasis. Altogether, these data suggest that as prostate cancer cells alter glucose and glutamine levels, O-GlcNAc modifications and OGT levels become elevated and are required for regulation of malignant properties, implicating OGT as a novel therapeutic target in the treatment of cancer.
Figures



Similar articles
-
Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1.Oncogene. 2010 May 13;29(19):2831-42. doi: 10.1038/onc.2010.41. Epub 2010 Mar 1. Oncogene. 2010. PMID: 20190804
-
O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway.Oncogene. 2017 Jan 26;36(4):559-569. doi: 10.1038/onc.2016.228. Epub 2016 Jun 27. Oncogene. 2017. PMID: 27345396 Free PMC article.
-
mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer.Mol Cancer Res. 2015 May;13(5):923-33. doi: 10.1158/1541-7786.MCR-14-0536. Epub 2015 Jan 30. Mol Cancer Res. 2015. PMID: 25636967 Free PMC article.
-
O-GlcNAcylation in cancer development and immunotherapy.Cancer Lett. 2023 Jul 10;566:216258. doi: 10.1016/j.canlet.2023.216258. Epub 2023 Jun 4. Cancer Lett. 2023. PMID: 37279852 Review.
-
Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation.Adv Cancer Res. 2015;126:137-66. doi: 10.1016/bs.acr.2014.12.003. Epub 2015 Feb 7. Adv Cancer Res. 2015. PMID: 25727147 Review.
Cited by
-
Cracking the O-GlcNAc code in metabolism.Trends Endocrinol Metab. 2013 Jun;24(6):301-9. doi: 10.1016/j.tem.2013.02.002. Epub 2013 May 4. Trends Endocrinol Metab. 2013. PMID: 23647930 Free PMC article. Review.
-
Mass Spectrometry-Based Glycoproteomic Workflows for Cancer Biomarker Discovery.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221148811. doi: 10.1177/15330338221148811. Technol Cancer Res Treat. 2023. PMID: 36740994 Free PMC article. Review.
-
Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer.J Biol Chem. 2018 Sep 7;293(36):13989-14000. doi: 10.1074/jbc.RA118.004709. Epub 2018 Jul 23. J Biol Chem. 2018. PMID: 30037904 Free PMC article.
-
Cancer metabolism and elevated O-GlcNAc in oncogenic signaling.J Biol Chem. 2014 Dec 12;289(50):34457-65. doi: 10.1074/jbc.R114.577718. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336642 Free PMC article. Review.
-
Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma.Oncotarget. 2014 Aug 30;5(16):6687-700. doi: 10.18632/oncotarget.2235. Oncotarget. 2014. PMID: 25149532 Free PMC article.
References
-
- Dang C. V., Semenza G. L. (1999) Oncogenic alterations of metabolism. Trends Biochem. Sci. 24, 68–72 - PubMed
-
- Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 - PubMed
-
- Kim J. W., Dang C. V. (2006) Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 66, 8927–8930 - PubMed
-
- Gatenby R. A. (1995) Models of tumor-host interaction as competing populations: implications for tumor biology and treatment. J. Theor. Biol. 176, 447–455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

