Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance
- PMID: 22275361
- PMCID: PMC3323042
- DOI: 10.1074/jbc.M111.317453
Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance
Abstract
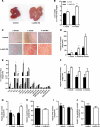
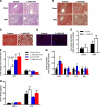
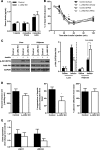
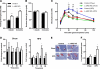
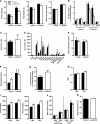
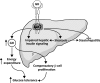
Non-alcoholic fatty liver disease (NAFLD) is becoming the leading cause of chronic liver disease and is now considered to be the hepatic manifestation of the metabolic syndrome. However, the role of steatosis per se and the precise factors required in the progression to steatohepatitis or insulin resistance remain elusive. The JAK-STAT pathway is critical in mediating signaling of a wide variety of cytokines and growth factors. Mice with hepatocyte-specific deletion of Janus kinase 2 (L-JAK2 KO mice) develop spontaneous steatosis as early as 2 weeks of age. In this study, we investigated the metabolic consequences of jak2 deletion in response to diet-induced metabolic stress. To our surprise, despite the profound hepatosteatosis, deletion of hepatic jak2 did not sensitize the liver to accelerated inflammatory injury on a prolonged high fat diet (HFD). This was accompanied by complete protection against HFD-induced whole-body insulin resistance and glucose intolerance. Improved glucose-stimulated insulin secretion and an increase in β-cell mass were also present in these mice. Moreover, L-JAK2 KO mice had progressively reduced adiposity in association with blunted hepatic growth hormone signaling. These mice also exhibited increased resting energy expenditure on both chow and high fat diet. In conclusion, our findings indicate a key role of hepatic JAK2 in metabolism such that its absence completely arrests steatohepatitis development and confers protection against diet-induced systemic insulin resistance and glucose intolerance.
Figures

References
-
- Angulo P. (2002) Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 - PubMed
-
- Szczepaniak L. S., Nurenberg P., Leonard D., Browning J. D., Reingold J. S., Grundy S., Hobbs H. H., Dobbins R. L. (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 288, E462–468 - PubMed
-
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A. J., Natale S., Forlani G., Melchionda N. (2001) Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50, 1844–1850 - PubMed
-
- Matteoni C. A., Younossi Z. M., Gramlich T., Boparai N., Liu Y. C., McCullough A. J. (1999) Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116, 1413–1419 - PubMed
-
- Younossi Z. M., Gramlich T., Liu Y. C., Matteoni C., Petrelli M., Goldblum J., Rybicki L., McCullough A. J. (1998) Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod. Pathol. 11, 560–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

