Insulin promotes neuronal survival via the alternatively spliced protein kinase CδII isoform
- PMID: 22275369
- PMCID: PMC3308802
- DOI: 10.1074/jbc.M111.313080
Insulin promotes neuronal survival via the alternatively spliced protein kinase CδII isoform
Abstract
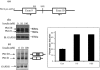
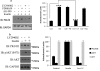
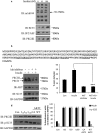
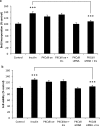
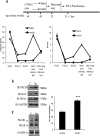
Insulin signaling pathways in the brain regulate food uptake and memory and learning. Insulin and protein kinase C (PKC) pathways are integrated and function closely together. PKC activation in the brain is essential for learning and neuronal repair. Intranasal delivery of insulin to the central nervous system (CNS) has been shown to improve memory, reduce cerebral atrophy, and reverse neurodegeneration. However, the neuronal molecular mechanisms of these effects have not been studied in depth. PKCδ plays a central role in cell survival. Its splice variants, PKCδI and PKCδII, are switches that determine cell survival and fate. PKCδI promotes apoptosis, whereas PKCδII promotes survival. Here, we demonstrate that insulin promotes alternative splicing of PKCδII isoform in HT22 cells. The expression of PKCδI splice variant remains unchanged. Insulin increases PKCδII alternative splicing via the PI3K pathway. We further demonstrate that Akt kinase mediates phosphorylation of the splicing factor SC35 to promote PKCδII alternative splicing. Using overexpression and knockdown assays, we demonstrate that insulin increases expression of Bcl2 and bcl-xL via PKCδII. We demonstrate increased cell proliferation and increased BrdU incorporation in insulin-treated cells as well as in HT22 cells overexpressing PKCδII. Finally, we demonstrate in vivo that intranasal insulin promotes cognitive function in mice with concomitant increases in PKCδII expression in the hippocampus. This is the first report of insulin, generally considered a growth or metabolic hormone, regulating the alternative isoform expression of a key signaling kinase in neuronal cells such that it results in increased neuronal survival.
Figures
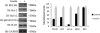
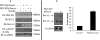

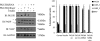
Similar articles
-
MALAT1 in Human Adipose Stem Cells Modulates Survival and Alternative Splicing of PKCδII in HT22 Cells.Endocrinology. 2017 Jan 1;158(1):183-195. doi: 10.1210/en.2016-1819. Endocrinology. 2017. PMID: 27841943 Free PMC article.
-
Protein kinase C δ (PKCδ) splice variants modulate apoptosis pathway in 3T3L1 cells during adipogenesis: identification of PKCδII inhibitor.J Biol Chem. 2013 Sep 13;288(37):26834-46. doi: 10.1074/jbc.M113.482638. Epub 2013 Jul 31. J Biol Chem. 2013. PMID: 23902767 Free PMC article.
-
PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells.Gene Expr. 2006;13(2):73-84. doi: 10.3727/000000006783991890. Gene Expr. 2006. PMID: 17017122 Free PMC article.
-
Vitamin A metabolite, all-trans-retinoic acid, mediates alternative splicing of protein kinase C deltaVIII (PKCdeltaVIII) isoform via splicing factor SC35.J Biol Chem. 2010 Aug 20;285(34):25987-95. doi: 10.1074/jbc.M110.100735. Epub 2010 Jun 14. J Biol Chem. 2010. PMID: 20547768 Free PMC article.
-
My road to alternative splicing control: from simple paths to loops and interconnections.Biochem Cell Biol. 2015 Jun;93(3):171-9. doi: 10.1139/bcb-2014-0161. Epub 2015 Feb 12. Biochem Cell Biol. 2015. PMID: 25759250 Review.
Cited by
-
Multifunctional roles of PKCδ: Opportunities for targeted therapy in human disease.Pharmacol Ther. 2016 Sep;165:1-13. doi: 10.1016/j.pharmthera.2016.05.001. Epub 2016 May 11. Pharmacol Ther. 2016. PMID: 27179744 Free PMC article. Review.
-
Is Glaucoma a Neurodegeneration caused by Central Insulin Resistance: Diabetes Type 4?J Curr Glaucoma Pract. 2017 Sep-Dec;11(3):77-79. doi: 10.5005/jp-journals-10028-1228. Epub 2017 Oct 27. J Curr Glaucoma Pract. 2017. PMID: 29151680 Free PMC article.
-
Falling Short: The Contribution of Central Insulin Receptors to Gait Dysregulation in Brain Aging.Biomedicines. 2022 Aug 9;10(8):1923. doi: 10.3390/biomedicines10081923. Biomedicines. 2022. PMID: 36009470 Free PMC article. Review.
-
Transformer 2β homolog (Drosophila) (TRA2B) regulates protein kinase C δI (PKCδI) splice variant expression during 3T3L1 preadipocyte cell cycle.J Biol Chem. 2014 Nov 14;289(46):31662-31672. doi: 10.1074/jbc.M114.592337. Epub 2014 Sep 26. J Biol Chem. 2014. PMID: 25261467 Free PMC article.
-
Intranasal insulin in Alzheimer's disease: Food for thought.Neuropharmacology. 2018 Jul 1;136(Pt B):196-201. doi: 10.1016/j.neuropharm.2017.11.037. Epub 2017 Nov 24. Neuropharmacology. 2018. PMID: 29180222 Free PMC article. Review.
References
-
- Benedict C., Hallschmid M., Schmitz K., Schultes B., Ratter F., Fehm H. L., Born J., Kern W. (2007) Intranasal insulin improves memory in humans. Superiority of insulin aspart. Neuropsychopharmacology 32, 239–243 - PubMed
-
- Schmidt H., Kern W., Giese R., Hallschmid M., Enders A. (2009) Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome. An exploratory clinical trial. J. Med. Genet. 46, 217–222 - PubMed
-
- Park C. R., Seeley R. J., Craft S., Woods S. C. (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 68, 509–514 - PubMed
-
- Francis G. J., Martinez J. A., Liu W. Q., Xu K., Ayer A., Fine J., Tuor U. I., Glazner G., Hanson L. R., Frey W. H., 2nd, Toth C. (2008) Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 131, 3311–3334 - PubMed
-
- Francis G. J., Martinez J. A., Liu W. Q., Xu K., Ayer A., Fine J., Tuor U. I., Glazner G., Hanson L. R., Frey W. H., 2nd, Toth C. (2008) Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 131, 3311–3334 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

