A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression
- PMID: 22275376
- PMCID: PMC3318694
- DOI: 10.1074/jbc.M111.286203
A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression
Abstract
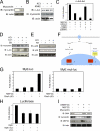
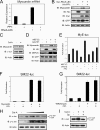
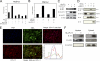
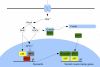
Differentiation of vascular smooth muscle cells (VSMC) is a fundamental aspect of normal development and vascular disease. During contraction, VSMCs modulate calcium sensitivity through RhoA/ROCK-mediated inhibition of the myosin light chain phosphatase complex (MLCP). Previous studies have demonstrated that this signaling pathway functions in parallel to increase the expression of smooth muscle genes through the myocardin-family of co-activators. MEF2C fulfills a critical role in VSMC differentiation and regulates myocardin expression, leading us to investigate whether the RhoA/ROCK signaling cascade might regulate MEF2 activity. Depolarization-induced calcium signaling increased the expression of myocardin, which was sensitive to ROCK and p38 MAPK inhibition. We previously identified protein phosphatase 1α (PP1α), a known catalytic subunit of the MLCP in VSMCs, as a potent repressor of MEF2 activity. PP1α inhibition resulted in increased expression of myocardin, while ectopic expression of PP1α inhibited the induction of myocardin by MEF2C. Consistent with these data, shRNA-mediated suppression of a PP1α inhibitor, CPI-17, reduced myocardin expression and inhibited VSMC differentiation, suggesting a pivotal role for CPI-17 in regulating MEF2 activity. These data constitute evidence of a novel signaling cascade that links RhoA-mediated calcium sensitivity to MEF2-dependent myocardin expression in VSMCs through a mechanism involving p38 MAPK, PP1α, and CPI-17.
Figures


Similar articles
-
Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2012 Jul 1;303(1):C58-68. doi: 10.1152/ajpcell.00118.2012. Epub 2012 Apr 25. Am J Physiol Cell Physiol. 2012. PMID: 22538237 Free PMC article.
-
Distinct effects of voltage- and store-dependent calcium influx on stretch-induced differentiation and growth in vascular smooth muscle.J Biol Chem. 2010 Oct 8;285(41):31829-39. doi: 10.1074/jbc.M109.097576. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675376 Free PMC article.
-
Mechanical stretch upregulates proteins involved in Ca2+ sensitization in urinary bladder smooth muscle hypertrophy.Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C542-53. doi: 10.1152/ajpcell.00033.2014. Epub 2014 Jul 16. Am J Physiol Cell Physiol. 2014. PMID: 25031021 Free PMC article.
-
Regulating a master regulator: establishing tissue-specific gene expression in skeletal muscle.Epigenetics. 2010 Nov-Dec;5(8):691-5. doi: 10.4161/epi.5.8.13045. Epub 2010 Nov 1. Epigenetics. 2010. PMID: 20716948 Free PMC article. Review.
-
Rho/ROCK-MYOCD in regulating airway smooth muscle growth and remodeling.Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L1-L5. doi: 10.1152/ajplung.00034.2021. Epub 2021 Apr 28. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33909498 Review.
Cited by
-
BNIP3L/Nix-induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation.Autophagy. 2021 Sep;17(9):2257-2272. doi: 10.1080/15548627.2020.1821548. Epub 2020 Oct 12. Autophagy. 2021. PMID: 33044904 Free PMC article.
-
Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2012 Jul 1;303(1):C58-68. doi: 10.1152/ajpcell.00118.2012. Epub 2012 Apr 25. Am J Physiol Cell Physiol. 2012. PMID: 22538237 Free PMC article.
-
ROCK inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1 expression and monocyte-endothelial cell adhesion.Cardiovasc Diabetol. 2012 Jun 13;11:65. doi: 10.1186/1475-2840-11-65. Cardiovasc Diabetol. 2012. PMID: 22694757 Free PMC article. Clinical Trial.
-
Mfge8 is expressed by pericytes in gastric antrum submucosa from patients with obesity.Am J Physiol Cell Physiol. 2023 May 1;324(5):C992-C1006. doi: 10.1152/ajpcell.00043.2023. Epub 2023 Mar 20. Am J Physiol Cell Physiol. 2023. PMID: 36939201 Free PMC article.
-
Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo.J Cell Sci. 2014 Jun 15;127(Pt 12):2627-38. doi: 10.1242/jcs.134833. Epub 2014 Apr 16. J Cell Sci. 2014. PMID: 24741067 Free PMC article.
References
-
- Drake C. J., Hungerford J. E., Little C. D. (1998) Morphogenesis of the first blood vessels. Ann. NY Acad. Sci. 857, 155–179 - PubMed
-
- Owens G. K. (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75, 487–517 - PubMed
-
- Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 - PubMed
-
- Miano J. M. (2003) Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 35, 577–593 - PubMed
-
- Creemers E. E., Sutherland L. B., McAnally J., Richardson J. A., Olson E. N. (2006) Myocardin is a direct transcriptional target of Mef2, Tead, and Foxo proteins during cardiovascular development. Development 133, 4245–4256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

