Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector
- PMID: 22275401
- PMCID: PMC3274376
- DOI: 10.1093/infdis/jir850
Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector
Abstract
Background: Vaccine development in human Plasmodium falciparum malaria has been hampered by the exceptionally high levels of CD8(+) T cells required for efficacy. Use of potently immunogenic human adenoviruses as vaccine vectors could overcome this problem, but these are limited by preexisting immunity to human adenoviruses.
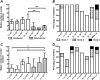
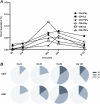
Methods: From 2007 to 2010, we undertook a phase I dose and route finding study of a new malaria vaccine, a replication-incompetent chimpanzee adenovirus 63 (ChAd63) encoding the preerythrocytic insert multiple epitope thrombospondin-related adhesion protein (ME-TRAP; n = 54 vaccinees) administered alone (n = 28) or with a modified vaccinia virus Ankara (MVA) ME-TRAP booster immunization 8 weeks later (n = 26). We observed an excellent safety profile. High levels of TRAP antigen-specific CD8(+) and CD4(+) T cells, as detected by interferon γ enzyme-linked immunospot assay and flow cytometry, were induced by intramuscular ChAd63 ME-TRAP immunization at doses of 5 × 10(10) viral particles and above. Subsequent administration of MVA ME-TRAP boosted responses to exceptionally high levels, and responses were maintained for up to 30 months postvaccination.
Conclusions: The ChAd63 chimpanzee adenovirus vector appears safe and highly immunogenic, providing a viable alternative to human adenoviruses as vaccine vectors for human use.
Clinical trials registration: NCT00890019.
Figures



References
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–2. - PubMed
-
- Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

