Structure-function relationship of the Polo-like kinase in Trypanosoma brucei
- PMID: 22275435
- PMCID: PMC3336379
- DOI: 10.1242/jcs.094243
Structure-function relationship of the Polo-like kinase in Trypanosoma brucei
Abstract
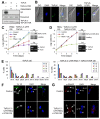
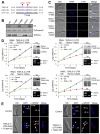
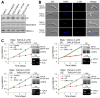
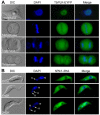
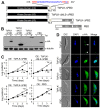
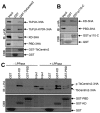
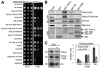
Polo-like kinases (Plks) play multiple roles in mitosis and cytokinesis in eukaryotes and are characterized by the C-terminal Polo-box domain (PBD), which is implicated in binding to Plk substrates, targeting Plk and regulating Plk activity. The Plk homolog in Trypanosoma brucei (TbPLK) possesses a similar architecture, but it lacks the crucial residues involved in substrate binding and regulates cytokinesis but not mitosis. Little is known about the regulation of TbPLK and the role of the PBD in TbPLK localization and function. Here, we addressed the requirement of the kinase activity and the PBD for TbPLK localization and function through coupling RNAi of endogenous TbPLK with ectopic expression of TbPLK mutants. We demonstrate that the kinase activity and phosphorylation of two threonine residues, Thr198 and Thr202, in the activation loop (T-loop) of the kinase domain are essential for TbPLK function but not for TbPLK localization. Deletion of the PBD abolishes TbPLK localization, but the PBD itself is not correctly targeted, indicating that TbPLK localization requires both the PBD and the kinase domain. Surprisingly, the kinase domain of TbPLK, but not the PBD, binds to its substrates TbCentrin2 and p110, suggesting that TbPLK might interact with its substrate through different mechanisms. Finally, the PBD interacts with the kinase domain of TbPLK and inhibits its activity, and this inhibition is relieved when Thr198 is phosphorylated. Together, these results suggest an essential role of T-loop phosphorylation in TbPLK activation and crucial roles of the PBD in regulating TbPLK activity and localization.
Figures
Similar articles
-
A Novel Basal Body Protein That Is a Polo-like Kinase Substrate Is Required for Basal Body Segregation and Flagellum Adhesion in Trypanosoma brucei.J Biol Chem. 2015 Oct 9;290(41):25012-22. doi: 10.1074/jbc.M115.674796. Epub 2015 Aug 13. J Biol Chem. 2015. PMID: 26272611 Free PMC article.
-
The structural basis of localizing polo-like kinase to the flagellum attachment zone in Trypanosoma brucei.PLoS One. 2011;6(11):e27303. doi: 10.1371/journal.pone.0027303. Epub 2011 Nov 11. PLoS One. 2011. PMID: 22096549 Free PMC article.
-
Polo-like kinase is expressed in S/G2/M phase and associated with the flagellum attachment zone in both procyclic and bloodstream forms of Trypanosoma brucei.Eukaryot Cell. 2008 Sep;7(9):1582-90. doi: 10.1128/EC.00150-08. Epub 2008 Jul 11. Eukaryot Cell. 2008. PMID: 18621923 Free PMC article.
-
Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy.Semin Cancer Biol. 2019 Jun;56:47-55. doi: 10.1016/j.semcancer.2017.11.004. Epub 2017 Nov 6. Semin Cancer Biol. 2019. PMID: 29122685 Review.
-
Understanding the Polo Kinase machine.Oncogene. 2015 Sep 10;34(37):4799-807. doi: 10.1038/onc.2014.451. Epub 2015 Jan 26. Oncogene. 2015. PMID: 25619835 Review.
Cited by
-
CRL4WDR1 Controls Polo-like Kinase Protein Abundance to Promote Bilobe Duplication, Basal Body Segregation and Flagellum Attachment in Trypanosoma brucei.PLoS Pathog. 2017 Jan 4;13(1):e1006146. doi: 10.1371/journal.ppat.1006146. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28052114 Free PMC article.
-
Proteomic identification of novel cytoskeletal proteins associated with TbPLK, an essential regulator of cell morphogenesis in Trypanosoma brucei.Mol Biol Cell. 2015 Sep 1;26(17):3013-29. doi: 10.1091/mbc.E15-04-0219. Epub 2015 Jul 1. Mol Biol Cell. 2015. PMID: 26133384 Free PMC article.
-
Functional analyses of the CIF1-CIF2 complex in trypanosomes identify the structural motifs required for cytokinesis.J Cell Sci. 2017 Dec 15;130(24):4108-4119. doi: 10.1242/jcs.207134. Epub 2017 Oct 26. J Cell Sci. 2017. PMID: 29074577 Free PMC article.
-
A Novel Basal Body Protein That Is a Polo-like Kinase Substrate Is Required for Basal Body Segregation and Flagellum Adhesion in Trypanosoma brucei.J Biol Chem. 2015 Oct 9;290(41):25012-22. doi: 10.1074/jbc.M115.674796. Epub 2015 Aug 13. J Biol Chem. 2015. PMID: 26272611 Free PMC article.
-
A DNA polymerization-independent role for mitochondrial DNA polymerase I-like protein C in African trypanosomes.J Cell Sci. 2020 May 7;133(9):jcs233072. doi: 10.1242/jcs.233072. J Cell Sci. 2020. PMID: 32079654 Free PMC article.
References
-
- Archambault V., Glover D. M. (2009). Polo-like kinases: conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10, 265-275 - PubMed
-
- Barr F. A., Sillje H. H., Nigg E. A. (2004). Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5, 429-440 - PubMed
-
- Briggs L. J., McKean P. G., Baines A., Moreira-Leite F., Davidge J., Vaughan S., Gull K. (2004). The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J. Cell Sci. 117, 1641-1651 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

