Loss of PKBβ/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo
- PMID: 22275470
- PMCID: PMC3339834
- DOI: 10.1242/dmm.008136
Loss of PKBβ/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo
Abstract
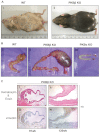
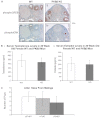
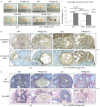
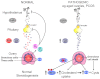
Ovarian cysts affect women of all ages and decrease fertility. In particular, polycystic ovarian syndrome (PCOS), in which multiple follicular cysts develop, affects 5-10% of women of reproductive age and can result in infertility. Current non-invasive treatments for PCOS can resolve cysts and restore fertility, but unresponsive patients must undergo severe ovarian wedge resection and resort to in vitro fertilization. PCOS is related to the deregulation of leutinizing hormone (LH) signaling at various levels of the hypothalamic-pituitary-ovarian axis and resultant hyperproduction of androgens. Because insulin resistance and compensatory hyperinsulinemia are observed in 50-70% of individuals with PCOS, deregulated insulin signaling in the ovary is considered an important factor in the disease. Here we report that aged mice specifically lacking the PKBβ (also known as Akt2) isoform that is crucial for insulin signaling develop increased testosterone levels and ovarian cysts, both of which are also observed in insulin-resistant PCOS patients. Young PKBβ knockout mice were used to model PCOS by treatment with LH and exhibited a cyst area that was threefold greater than in controls, but without hyperinsulinemia. Thus, loss of PKBβ might predispose mice to ovarian cysts independently of hyperactive insulin signaling. Targeted therapeutic augmentation of specific PKBβ signaling could therefore provide a new avenue for the treatment and management of ovarian cysts.
Figures

Similar articles
-
Liuwei Dihuang Pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/Akt signaling pathway.J Ethnopharmacol. 2020 Mar 25;250:111965. doi: 10.1016/j.jep.2019.111965. Epub 2019 Jun 8. J Ethnopharmacol. 2020. PMID: 31185267
-
Selective deletion of Pten in theca-interstitial cells leads to androgen excess and ovarian dysfunction in mice.Mol Cell Endocrinol. 2017 Mar 15;444:26-37. doi: 10.1016/j.mce.2017.01.043. Epub 2017 Jan 28. Mol Cell Endocrinol. 2017. PMID: 28137614
-
Thecal cell sensitivity to luteinizing hormone and insulin in polycystic ovarian syndrome.Reprod Biol. 2016 Mar;16(1):53-60. doi: 10.1016/j.repbio.2015.12.006. Epub 2016 Jan 13. Reprod Biol. 2016. PMID: 26952754
-
Steroidogenesis in human polycystic ovary.Endocrinol Metab Clin North Am. 1988 Dec;17(4):751-69. Endocrinol Metab Clin North Am. 1988. PMID: 3143567 Review.
-
Molecular Mechanisms of Laparoscopic Ovarian Drilling and Its Therapeutic Effects in Polycystic Ovary Syndrome.Int J Mol Sci. 2020 Oct 31;21(21):8147. doi: 10.3390/ijms21218147. Int J Mol Sci. 2020. PMID: 33142702 Free PMC article. Review.
Cited by
-
Hepatic proteomic analysis revealed altered metabolic pathways in insulin resistant Akt1(+/-)/Akt2(-/-) mice.Metabolism. 2015 Dec;64(12):1694-703. doi: 10.1016/j.metabol.2015.09.008. Epub 2015 Sep 12. Metabolism. 2015. PMID: 26455965 Free PMC article.
-
Chamomile and Urtica dioica extracts improve immunological and histological alterations associated with polycystic ovarian syndrome in DHEA -induced mice.BMC Complement Med Ther. 2023 Apr 3;23(1):102. doi: 10.1186/s12906-023-03936-7. BMC Complement Med Ther. 2023. PMID: 37013510 Free PMC article.
-
Genetic Rodent Models of Obesity-Associated Ovarian Dysfunction and Subfertility: Insights into Polycystic Ovary Syndrome.Front Endocrinol (Lausanne). 2016 Jun 7;7:53. doi: 10.3389/fendo.2016.00053. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27375552 Free PMC article. Review.
-
Molecular crosstalk between insulin-like growth factors and follicle-stimulating hormone in the regulation of granulosa cell function.Reprod Med Biol. 2024 Apr 3;23(1):e12575. doi: 10.1002/rmb2.12575. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 38571513 Free PMC article. Review.
-
Fractalkine restores the decreased expression of StAR and progesterone in granulosa cells from patients with polycystic ovary syndrome.Sci Rep. 2016 Jul 8;6:26205. doi: 10.1038/srep26205. Sci Rep. 2016. PMID: 27386819 Free PMC article.
References
-
- Bogovich K. (1987). Induction of follicular cysts in rat ovaries by prolonged administration of human chorionic gonadotropin. Adv. Exp. Med. Biol. 219, 659–663 - PubMed
-
- Bogovich K., Richards J. S. (1982). Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology 111, 1201–1208 - PubMed
-
- Chang R. J. (2007). The reproductive phenotype in polycystic ovary syndrome. Nat. Clin. Pract. Endocrinol. Metab. 3, 688–695 - PubMed
-
- Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. (2001a). Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 - PubMed
-
- Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001b). Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

