Polysialylated NCAM and ephrinA/EphA regulate synaptic development of GABAergic interneurons in prefrontal cortex
- PMID: 22275477
- PMCID: PMC3513957
- DOI: 10.1093/cercor/bhr392
Polysialylated NCAM and ephrinA/EphA regulate synaptic development of GABAergic interneurons in prefrontal cortex
Abstract
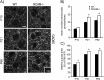
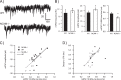
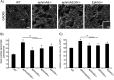
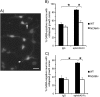
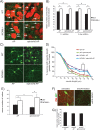
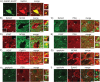
A novel function for the neural cell adhesion molecule (NCAM) was identified in ephrinA/EphA-mediated repulsion as an important regulatory mechanism for development of GABAergic inhibitory synaptic connections in mouse prefrontal cortex. Deletion of NCAM, EphA3, or ephrinA2/3/5 in null mutant mice increased the numbers and size of perisomatic synapses between GABAergic basket interneurons and pyramidal cells in the developing cingulate cortex (layers II/III). A functional consequence of NCAM loss was increased amplitudes and faster kinetics of miniature inhibitory postsynaptic currents in NCAM null cingulate cortex. NCAM and EphA3 formed a molecular complex and colocalized with the inhibitory presynaptic marker vesicular GABA transporter (VGAT) in perisomatic puncta and neuropil in the cingulate cortex. EphrinA5 treatment promoted axon remodeling of enhanced green fluorescent protein-labeled basket interneurons in cortical slice cultures and induced growth cone collapse in wild-type but not NCAM null mutant neurons. NCAM modified with polysialic acid (PSA) was required to promote ephrinA5-induced axon remodeling of basket interneurons in cortical slices, likely by providing a permissive environment for ephrinA5/EphA3 signaling. These results reveal a new mechanism in which NCAM and ephrinAs/EphA3 coordinate to constrain GABAergic interneuronal arborization and perisomatic innervation, potentially contributing to excitatory/inhibitory balance in prefrontal cortical circuitry.
Figures


References
-
- Aisa B, Gil-Bea FJ, Solas M, Garcia-Alloza M, Chen CP, Lai MK, Francis PT, Ramirez MJ. Altered NCAM expression associated with the cholinergic system in Alzheimer's disease. J Alzheimers Dis. 2010;20:659–668. - PubMed
-
- Angata K, Long JM, Bukalo O, Lee W, Dityatev A, Wynshaw-Boris A, Schachner M, Fukuda M, Marth JD. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem. 2004;279:32603–32613. - PubMed
-
- Arai M, Yamada K, Toyota T, Obata N, Haga S, Yoshida Y, Nakamura K, Minabe Y, Ujike H, Sora I, et al. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol Psychiatry. 2006;59:652–659. - PubMed
-
- Arnall S, Cheam LY, Smart C, Rengel A, Fitzgerald M, Thivierge JP, Rodger J. Abnormal strategies during visual discrimination reversal learning in ephrin-A2 (-/-) mice. Behav Brain Res. 2010;209:109–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

