Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats
- PMID: 22275486
- PMCID: PMC3303627
- DOI: 10.1161/CIRCRESAHA.111.258285
Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats
Abstract
Rationale: Hyperamylinemia is common in patients with obesity and insulin resistance, coincides with hyperinsulinemia, and results in amyloid deposition. Amylin amyloids are generally considered a pancreatic disorder in type 2 diabetes. However, elevated circulating levels of amylin may also lead to amylin accumulation and proteotoxicity in peripheral organs, including the heart.
Objective: To test whether amylin accumulates in the heart of obese and type 2 diabetic patients and to uncover the effects of amylin accumulation on cardiac morphology and function.
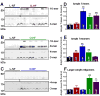
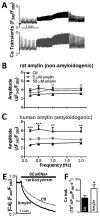
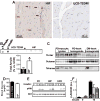
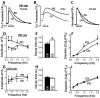
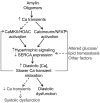
Methods and results: We compared amylin deposition in failing and nonfailing hearts from lean, obese, and type 2 diabetic humans using immunohistochemistry and Western blots. We found significant accumulation of large amylin oligomers, fibrils, and plaques in failing hearts from obese and diabetic patients but not in normal hearts and failing hearts from lean, nondiabetic humans. Small amylin oligomers were even elevated in nonfailing hearts from overweight/obese patients, suggesting an early state of accumulation. Using a rat model of hyperamylinemia transgenic for human amylin, we observed that amylin oligomers attach to the sarcolemma, leading to myocyte Ca(2+) dysregulation, pathological myocyte remodeling, and diastolic dysfunction, starting from prediabetes. In contrast, prediabetic rats expressing the same level of wild-type rat amylin, a nonamyloidogenic isoform, exhibited normal heart structure and function.
Conclusions: Hyperamylinemia promotes amylin deposition in the heart, causing alterations of cardiac myocyte structure and function. We propose that detection and disruption of cardiac amylin buildup may be both a predictor of heart dysfunction and a novel therapeutic strategy in diabetic cardiomyopathy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- HL075675/HL/NHLBI NIH HHS/United States
- P01 HL080101/HL/NHLBI NIH HHS/United States
- R01-AG017022/AG/NIA NIH HHS/United States
- R01-HL079071/HL/NHLBI NIH HHS/United States
- R01-HL061483/HL/NHLBI NIH HHS/United States
- R01-HL073162/HL/NHLBI NIH HHS/United States
- R01 AG017022/AG/NIA NIH HHS/United States
- R01 HL077281/HL/NHLBI NIH HHS/United States
- R21 AT003645/AT/NCCIH NIH HHS/United States
- R01 HL073162/HL/NHLBI NIH HHS/United States
- R01 HL109501/HL/NHLBI NIH HHS/United States
- HL091333/HL/NHLBI NIH HHS/United States
- R01 HL079071/HL/NHLBI NIH HHS/United States
- R01-HL077281/HL/NHLBI NIH HHS/United States
- RC1 DK087307/DK/NIDDK NIH HHS/United States
- R01-HL109501/HL/NHLBI NIH HHS/United States
- HL107256/HL/NHLBI NIH HHS/United States
- R01 HL089847/HL/NHLBI NIH HHS/United States
- R01 HL075675/HL/NHLBI NIH HHS/United States
- R01-HL089847/HL/NHLBI NIH HHS/United States
- R01 HL107256/HL/NHLBI NIH HHS/United States
- R01 HL061483/HL/NHLBI NIH HHS/United States
- AT003645/AT/NCCIH NIH HHS/United States
- R01 HL091333/HL/NHLBI NIH HHS/United States
- K08 HL003560/HL/NHLBI NIH HHS/United States
- DK087307/DK/NIDDK NIH HHS/United States
- R37 HL030077/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

