Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells
- PMID: 22275487
- PMCID: PMC3292662
- DOI: 10.1161/CIRCRESAHA.111.259507
Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells
Retraction in
-
Retraction of: Cardiomyogenesis in the Developing Heart Is Regulated by C-Kit-Positive Cardiac Stem Cells.Circ Res. 2019 Feb 15;124(4):e28. doi: 10.1161/RES.0000000000000252. Circ Res. 2019. PMID: 30582467 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
Rationale: Embryonic and fetal myocardial growth is characterized by a dramatic increase in myocyte number, but whether the expansion of the myocyte compartment is dictated by activation and commitment of resident cardiac stem cells (CSCs), division of immature myocytes or both is currently unknown.
Objective: In this study, we tested whether prenatal cardiac development is controlled by activation and differentiation of CSCs and whether division of c-kit-positive CSCs in the mouse heart is triggered by spontaneous Ca(2+) oscillations.
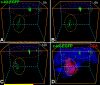
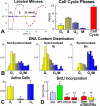
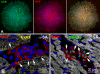
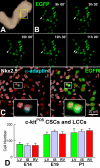
Methods and results: We report that embryonic-fetal c-kit-positive CSCs are self-renewing, clonogenic and multipotent in vitro and in vivo. The growth and commitment of c-kit-positive CSCs is responsible for the generation of the myocyte progeny of the developing heart. The close correspondence between values computed by mathematical modeling and direct measurements of myocyte number at E9, E14, E19 and 1 day after birth strongly suggests that the organogenesis of the embryonic heart is dependent on a hierarchical model of cell differentiation regulated by resident CSCs. The growth promoting effects of c-kit-positive CSCs are triggered by spontaneous oscillations in intracellular Ca(2+), mediated by IP3 receptor activation, which condition asymmetrical stem cell division and myocyte lineage specification.
Conclusions: Myocyte formation derived from CSC differentiation is the major determinant of cardiac growth during development. Division of c-kit-positive CSCs in the mouse is promoted by spontaneous Ca(2+) spikes, which dictate the pattern of stem cell replication and the generation of a myocyte progeny at all phases of prenatal life and up to one day after birth.
Figures

















Similar articles
-
Inhibition of notch1-dependent cardiomyogenesis leads to a dilated myopathy in the neonatal heart.Circ Res. 2010 Aug 6;107(3):429-41. doi: 10.1161/CIRCRESAHA.110.218487. Epub 2010 Jun 17. Circ Res. 2010. PMID: 20558824 Free PMC article.
-
Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts.Stem Cells Dev. 2010 Jan;19(1):105-16. doi: 10.1089/scd.2009.0041. Stem Cells Dev. 2010. PMID: 19580375
-
c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy.Circ Res. 2014 Jan 3;114(1):41-55. doi: 10.1161/CIRCRESAHA.114.302500. Epub 2013 Oct 29. Circ Res. 2014. PMID: 24170267 Free PMC article.
-
Cardiac stem cells and myocardial regeneration.Novartis Found Symp. 2005;265:142-54; discussion 155-7, 204-11. Novartis Found Symp. 2005. PMID: 16050255 Review.
-
Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration.Nat Clin Pract Cardiovasc Med. 2006 Mar;3 Suppl 1:S8-13. doi: 10.1038/ncpcardio0409. Nat Clin Pract Cardiovasc Med. 2006. PMID: 16501638 Review.
Cited by
-
Sca-1+ cardiac progenitor cells and heart-making: a critical synopsis.Stem Cells Dev. 2014 Oct 1;23(19):2263-73. doi: 10.1089/scd.2014.0197. Epub 2014 Jul 14. Stem Cells Dev. 2014. PMID: 24926741 Free PMC article. Review.
-
Novel direct reprogramming technique for the generation of culture-expandable cardiac progenitor cells from fibroblasts.Stem Cell Investig. 2017 Feb 16;4:15. doi: 10.21037/sci.2017.02.04. eCollection 2017. Stem Cell Investig. 2017. PMID: 28275645 Free PMC article. No abstract available.
-
Stem cell populations in the heart and the role of Isl1 positive cells.Eur J Histochem. 2013 May 9;57(2):e14. doi: 10.4081/ejh.2013.e14. Eur J Histochem. 2013. PMID: 23807293 Free PMC article.
-
Cardiac stem cells: biology and clinical applications.Antioxid Redox Signal. 2014 Nov 10;21(14):2002-17. doi: 10.1089/ars.2014.5875. Epub 2014 Apr 10. Antioxid Redox Signal. 2014. PMID: 24597850 Free PMC article. Review.
-
cKit+ cardiac progenitors of neural crest origin.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):13051-6. doi: 10.1073/pnas.1517201112. Epub 2015 Oct 5. Proc Natl Acad Sci U S A. 2015. PMID: 26438843 Free PMC article.
References
-
- Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17174. - PMC - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. - PMC - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, LeCapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

