9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema
- PMID: 22275501
- PMCID: PMC3327127
- DOI: 10.1161/CIRCULATIONAHA.111.030296
9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema
Erratum in
-
Correction. 9-Cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-Cis retinoic acid for secondary lymphedema.Circulation. 2015 Apr 7;131(14):e401-2. doi: 10.1161/CIR.0000000000000200. Circulation. 2015. PMID: 25847986 No abstract available.
Abstract
Background: The lymphatic system plays a key role in tissue fluid homeostasis and lymphatic dysfunction caused by genetic defects, or lymphatic vessel obstruction can cause lymphedema, disfiguring tissue swelling often associated with fibrosis and recurrent infections with no available cures to date. In this study, retinoic acids (RAs) were determined to be a potent therapeutic agent that is immediately applicable to reduce secondary lymphedema.
Methods and results: We report that RAs promote proliferation, migration, and tube formation of cultured lymphatic endothelial cells by activating fibroblast growth factor receptor signaling. Moreover, RAs control the expression of cell-cycle checkpoint regulators such as p27(Kip1), p57(Kip2), and the aurora kinases through both an Akt-mediated nongenomic action and a transcription-dependent genomic action that is mediated by Prox1, a master regulator of lymphatic development. Moreover, 9-cisRA was found to activate in vivo lymphangiogenesis in animals in mouse trachea, Matrigel plug, and cornea pocket assays. Finally, we demonstrate that 9-cisRA can provide a strong therapeutic efficacy in ameliorating experimental mouse tail lymphedema by enhancing lymphatic vessel regeneration.
Conclusion: These in vitro and animal studies demonstrate that 9-cisRA potently activates lymphangiogenesis and promotes lymphatic regeneration in an experimental lymphedema model, presenting it as a promising novel therapeutic agent to treat human lymphedema patients.
Conflict of interest statement
Figures
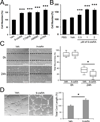
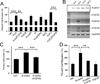
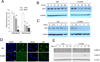

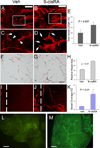
Comment in
-
Lymphangiogenesis: a potential new therapy for lymphedema?Circulation. 2012 Feb 21;125(7):853-5. doi: 10.1161/CIRCULATIONAHA.111.083477. Epub 2012 Jan 24. Circulation. 2012. PMID: 22275500 Free PMC article. No abstract available.
References
-
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. - PubMed
-
- Lahteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lahteenvuo J, Kholova I, Alitalo K, Yla-Herttuala S, Saaristo A. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation. 2011;123:613–620. - PubMed
-
- Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. - PubMed
-
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid x receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11 Suppl 2:S126–S143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

