Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant
- PMID: 22275811
- PMCID: PMC3261693
- DOI: 10.2147/OPTH.S24397
Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant
Abstract
Purpose: To describe visual acuity (VA) and inflammation following cataract surgery in eyes with noninfectious posterior uveitis (NIPU) that were being treated with a fluocinolone acetonide (FA) intravitreal implant compared with those that were not.
Design: Post hoc, subgroup analysis of data from a 3-year, dose-masked, randomized, multicenter trial evaluating the FA implant for the treatment of NIPU.
Participants and controls: The subset of eyes that underwent cataract surgery during the 3-year trial. Eyes were either implanted with a 0.59- or a 2.1-mg FA implant, or, in the case of affected fellow eyes, received standard-of-care local treatment.
Main outcome measures: VA, anterior and posterior chamber inflammation at 1 and 3 months after surgery, and rate of uveitis recurrence and serious postoperative ocular adverse events.
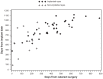
Results: Of 278 patients enrolled in the main trial, 132/142 phakic implanted eyes and 39/186 phakic non-implanted eyes underwent cataract surgery. Mean improvement in VA was significantly greater in implanted than non-implanted eyes at 1 (P = 0.0047) and 3 months (P = 0.0015) postoperatively; significantly fewer anterior chamber cells were seen in implanted than non-implanted eyes at 1 (P = 0.0084) and 3 months (P = 0.0002). Severity of vitreous haze was less in implanted than non-implanted eyes at 3 months postoperatively (P = 0.0005). The postsurgical uveitis recurrence rate was lower in implanted than non-implanted eyes (26.5% vs 44.4%; P = 0.0433). Glaucoma was reported in 19.7% of implanted eyes and no non-implanted eyes (P = 0.0008) postoperatively.
Conclusion: In this post hoc subgroup analysis, eyes with NIPU treated with the FA intravitreal implant demonstrated better vision and less intraocular inflammation following cataract surgery than non-implanted eyes. Recurrent uveitic inflammation did not appear to be triggered by cataract surgery. Glaucoma occurred more frequently in implanted eyes.
Keywords: cataract surgery; glaucoma; intraocular pressure; posterior chamber inflammation; steroid implant; visual acuity.
Figures
References
-
- Becker MD, Smith JR, Max R, Fiehn C. Management of sight-threatening uveitis: new therapeutic options. Drugs. 2005;65(4):497–519. - PubMed
-
- Callanan DG, Jaffe GJ, Martin DF, Pearson A, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant. Arch Ophthalmol. 2008;126(9):1191–1201. - PubMed
-
- Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107(11):2024–2033. - PubMed
-
- Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112(7):1192–1198. - PubMed
-
- Kimura SJ, Thygeson P, Hogan MJ. Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol. 1959;47(5 Part 2):171–176. - PubMed
LinkOut - more resources
Full Text Sources

