Bypassing multidrug resistance in human breast cancer cells with lipid/polymer particle assemblies
- PMID: 22275834
- PMCID: PMC3263411
- DOI: 10.2147/IJN.S27864
Bypassing multidrug resistance in human breast cancer cells with lipid/polymer particle assemblies
Abstract
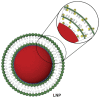
Background: Multidrug resistance (MDR) mediated by the overexpression of adenosine triphosphate (ATP)-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp), remains one of the major obstacles to effective cancer chemotherapy. In this study, lipid/particle assemblies named LipoParticles (LNPs), consisting of a dimethyldidodecylammonium bromide (DMAB)-modified poly(lactic-co-glycolic acid) (PLGA) nanoparticle core surrounded by a 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) shell, were specially designed for anticancer drugs to bypass MDR in human breast cancer cells that overexpress P-gp.
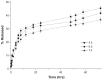
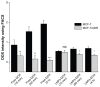
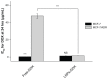
Methods: Doxorubicin (DOX), a chemotherapy drug that is a P-gp substrate, was conjugated to PLGA and encapsulated in the self-assembled LNP structure. Physiochemical properties of the DOX-loaded LNPs were characterized in vitro. Cellular uptake, intracellular accumulation, and cytotoxicity were compared in parental Michigan Cancer Foundation (MCF)-7 cells and P-gp-overexpressing, resistant MCF-7/adriamycin (MCF-7/ADR) cells.
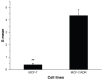
Results: This study found that the DOX formulated in LNPs showed a significantly increased accumulation in the nuclei of drug-resistant cells relative to the free drug, indicating that LNPs could alter intracellular traffic and bypass drug efflux. The cytotoxicity of DOX loaded-LNPs had a 30-fold lower half maximal inhibitory concentration (IC(50)) value than free DOX in MCF-7/ADR, measured by the colorimetric cell viability (MTT) assay, correlated with the strong nuclear retention of the drug.
Conclusion: The results show that this core-shell lipid/particle structure could be a promising strategy to bypass MDR.
Keywords: chemotherapy; drug delivery; multidrug resistance; polymeric nanoparticles.
Figures


Similar articles
-
Multifunctional PLGA Nanobubbles as Theranostic Agents: Combining Doxorubicin and P-gp siRNA Co-Delivery Into Human Breast Cancer Cells and Ultrasound Cellular Imaging.J Biomed Nanotechnol. 2015 Dec;11(12):2124-36. doi: 10.1166/jbn.2015.2168. J Biomed Nanotechnol. 2015. PMID: 26510307
-
An all-in-one nanoparticle for overcoming drug resistance: doxorubicin and elacridar co-loaded folate receptor targeted PLGA/MSN hybrid nanoparticles.J Drug Target. 2024 Nov;32(9):1101-1110. doi: 10.1080/1061186X.2024.2374034. Epub 2024 Jul 5. J Drug Target. 2024. PMID: 38946465
-
Novel nanostructured enoxaparin sodium-PLGA hybrid carriers overcome tumor multidrug resistance of doxorubicin hydrochloride.Int J Pharm. 2016 Nov 20;513(1-2):218-226. doi: 10.1016/j.ijpharm.2016.09.037. Epub 2016 Sep 11. Int J Pharm. 2016. PMID: 27628785
-
Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer.Nanomedicine. 2022 Feb;40:102494. doi: 10.1016/j.nano.2021.102494. Epub 2021 Nov 12. Nanomedicine. 2022. PMID: 34775061 Review.
-
Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities.Adv Drug Deliv Rev. 2013 Nov;65(13-14):1731-47. doi: 10.1016/j.addr.2013.09.001. Epub 2013 Sep 10. Adv Drug Deliv Rev. 2013. PMID: 24036273 Free PMC article. Review.
Cited by
-
Use of (99m)Tc-doxorubicin scintigraphy in females with breast cancer: a pilot study.Br J Radiol. 2015 Aug;88(1052):20150268. doi: 10.1259/bjr.20150268. Epub 2015 May 27. Br J Radiol. 2015. PMID: 26111270 Free PMC article.
-
Gap Junction Liposomes for Efficient Delivery of Chemotherapeutics to Solid Tumors.ACS Biomater Sci Eng. 2020 Sep 14;6(9):4851-4857. doi: 10.1021/acsbiomaterials.0c01047. Epub 2020 Aug 24. ACS Biomater Sci Eng. 2020. PMID: 33455217 Free PMC article.
-
Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery.Int J Nanomedicine. 2015 Mar 16;10:2101-14. doi: 10.2147/IJN.S77667. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25844039 Free PMC article.
-
Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer.J Nanobiotechnology. 2022 Sep 24;20(1):423. doi: 10.1186/s12951-022-01626-z. J Nanobiotechnology. 2022. PMID: 36153528 Free PMC article. Review.
-
Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches.Front Oncol. 2022 Jun 23;12:891652. doi: 10.3389/fonc.2022.891652. eCollection 2022. Front Oncol. 2022. PMID: 35814435 Free PMC article. Review.
References
-
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. - PubMed
-
- Kohno K, Sato S, Takano H, et al. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem Biophys Res Commun. 1989;165(3):1415–1421. - PubMed
-
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, et al. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22(47):7468–7485. - PubMed
-
- Fletcher JI, Haber M, Henderson MJ, et al. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

