In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation
- PMID: 22275835
- PMCID: PMC3263412
- DOI: 10.2147/IJN.S26763
In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation
Abstract
Background: A liquisolid technique has been reported to be a new approach to improve the release of poorly water-soluble drugs for oral administration. However, an apparent limitation of this technique is the formulation of a high dose because a large amount of liquid vehicle is needed, which finally results in a low-dose liquisolid formulation. Silica as an absorbent has been used extensively in liquisolid formulations. Although nanoparticle silica can be prepared and used to improve liquid adsorption capacity, loading a high dose of drug into a liquisolid is still a challenge. With the aim of improving adsorption capacity and accordingly achieving high drug loading, ordered mesoporous silica with a high surface area and narrow pore size distribution was synthesized and used in a liquisolid formulation.
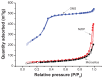
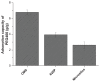
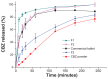
Methods: Ordered mesoporous silica was synthesized and its particle size and morphology were tailored by controlling the concentration of cetyltrimethyl ammonium bromide. The ordered mesoporous silica synthesized was characterized by transmission electron microscopy, scanning electron microscopy, Fourier transform infrared spectroscopy, small-angle x-ray diffraction, wide angle x-ray diffraction, and nitrogen adsorption-desorption measurements. The liquid adsorption capacity of ordered mesoporous silica was subsequently compared with that of conventional silica materials using PEG400 as the model liquid. Carbamazepine was chosen as a model drug to prepare the liquisolid formulation, with ordered mesoporous silica as the adsorbent material. The preparation was evaluated and compared with commercially available fast-release carbamazepine tablets in vitro and in vivo.
Results: Characterization of the ordered mesoporous silica synthesized in this study indicated a huge Brunauer-Emmett-Teller surface area (1030 m(2)/g), an ordered mesoporous structure with a pore size of 2.8 nm, and high adsorption capacity for liquid compared with conventional silica. Compared with fast-release commercial carbamazepine tablets, drug release from the liquisolid capsules was greatly improved, and the bioavailability of the liquisolid preparation was enhanced by 182.7%.
Conclusion: Ordered mesoporous silica is a potentially attractive adsorbent which may lead to a new approach for development of liquisolid products.
Keywords: bioavailability; carbamazepine; liquisolid; ordered mesoporous silica; poorly water-soluble drug.
Figures






Similar articles
-
Increasing the oral bioavailability of poorly water-soluble carbamazepine using immediate-release pellets supported on SBA-15 mesoporous silica.Int J Nanomedicine. 2012;7:5807-18. doi: 10.2147/IJN.S37650. Epub 2012 Nov 22. Int J Nanomedicine. 2012. PMID: 23209366 Free PMC article.
-
Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: a case study with aceclofenac.Int J Pharm. 2014 Jan 30;461(1-2):459-68. doi: 10.1016/j.ijpharm.2013.12.017. Epub 2013 Dec 22. Int J Pharm. 2014. PMID: 24368106
-
Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin.Eur J Pharm Sci. 2020 Jan 15;142:105103. doi: 10.1016/j.ejps.2019.105103. Epub 2019 Oct 21. Eur J Pharm Sci. 2020. PMID: 31648050
-
Mesoporous silica formulation strategies for drug dissolution enhancement: a review.Expert Opin Drug Deliv. 2016;13(1):93-108. doi: 10.1517/17425247.2016.1100165. Epub 2015 Nov 7. Expert Opin Drug Deliv. 2016. PMID: 26549623 Review.
-
[Enhancing of drug bioavailability using liquisolid system formulation].Ceska Slov Farm. 2015 Jun;64(3):55-66. Ceska Slov Farm. 2015. PMID: 26400228 Review. Czech.
Cited by
-
Preparation, characterization, and in vivo evaluation of tanshinone IIA solid dispersions with silica nanoparticles.Int J Nanomedicine. 2013;8:2285-93. doi: 10.2147/IJN.S40374. Epub 2013 Jun 25. Int J Nanomedicine. 2013. PMID: 23836971 Free PMC article.
-
Ordered nanoporous silica as carriers for improved delivery of water insoluble drugs: a comparative study between three dimensional and two dimensional macroporous silica.Int J Nanomedicine. 2013;8:4015-31. doi: 10.2147/IJN.S52605. Epub 2013 Oct 22. Int J Nanomedicine. 2013. PMID: 24174875 Free PMC article.
-
Mesoporous Silica Particles as Drug Delivery Systems-The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes.Pharmaceutics. 2021 Jun 24;13(7):950. doi: 10.3390/pharmaceutics13070950. Pharmaceutics. 2021. PMID: 34202794 Free PMC article. Review.
-
Solubility Enhanced Formulation Approaches to Overcome Oral Delivery Obstacles of PROTACs.Pharmaceutics. 2023 Jan 3;15(1):156. doi: 10.3390/pharmaceutics15010156. Pharmaceutics. 2023. PMID: 36678785 Free PMC article.
-
Multifunctional nanoparticles for real-time evaluation of toxicity during fetal development.PLoS One. 2018 Feb 8;13(2):e0192474. doi: 10.1371/journal.pone.0192474. eCollection 2018. PLoS One. 2018. PMID: 29420606 Free PMC article.
References
-
- Karmarkar AB, Gonjari ID, Hosmani AH. Liquisolid technology for dissolution rate enhancement or sustained release. Expert Opin Drug Deliv. 2010;7:1227–1234. - PubMed
-
- El-Houssieny BM, Wahman LF, Arafa NM. Bioavailability and biological activity of liquisolid compact formula of repaglinide and its effect on glucose tolerance in rabbits. Biosci Trends. 2010;4:17–24. - PubMed
-
- Nokhodchi A, Aliakbar R, Desai S, Javadzadeh Y. Liquisolid compacts: the effect of cosolvent and HPMC on theophylline release. Colloids Surf B Biointerfaces. 2010;79:262–269. - PubMed
-
- Tiong N, Elkordy AA. Effects of liquisolid formulations on dissolution of naproxen. Eur J Pharm Biopharm. 2009;73:373–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

