Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method
- PMID: 22275837
- PMCID: PMC3263414
- DOI: 10.2147/IJN.S27709
Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method
Abstract
Background: Low drug entrapment efficiency of hydrophilic drugs into poly(lactic-co-glycolic acid) (PLGA) nanoparticles is a major drawback. The objective of this work was to investigate different methods of producing PLGA nanoparticles containing minocycline, a drug suitable for periodontal infections.
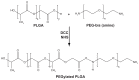
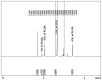
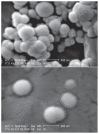
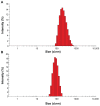
Methods: Different methods, such as single and double solvent evaporation emulsion, ion pairing, and nanoprecipitation were used to prepare both PLGA and PEGylated PLGA nanoparticles. The resulting nanoparticles were analyzed for their morphology, particle size and size distribution, drug loading and entrapment efficiency, thermal properties, and antibacterial activity.
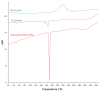
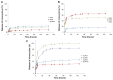
Results: The nanoparticles prepared in this study were spherical, with an average particle size of 85-424 nm. The entrapment efficiency of the nanoparticles prepared using different methods was as follows: solid/oil/water ion pairing (29.9%) > oil/oil (5.5%) > water/oil/water (4.7%) > modified oil/water (4.1%) > nano precipitation (0.8%). Addition of dextran sulfate as an ion pairing agent, acting as an ionic spacer between PEGylated PLGA and minocycline, decreased the water solubility of minocycline, hence increasing the drug entrapment efficiency. Entrapment efficiency was also increased when low molecular weight PLGA and high molecular weight dextran sulfate was used. Drug release studies performed in phosphate buffer at pH 7.4 indicated slow release of minocycline from 3 days to several weeks. On antibacterial analysis, the minimum inhibitory concentration and minimum bactericidal concentration of nanoparticles was at least two times lower than that of the free drug.
Conclusion: Novel minocycline-PEGylated PLGA nanoparticles prepared by the ion pairing method had the best drug loading and entrapment efficiency compared with other prepared nanoparticles. They also showed higher in vitro antibacterial activity than the free drug.
Keywords: PEGylation; PLGA; antibacterial; ion pairing; minocycline; nanoparticle.
Figures

Similar articles
-
Hydrophobic ion pairing of a minocycline/Ca(2+)/AOT complex for preparation of drug-loaded PLGA nanoparticles with improved sustained release.Int J Pharm. 2016 Feb 29;499(1-2):351-357. doi: 10.1016/j.ijpharm.2016.01.011. Epub 2016 Jan 7. Int J Pharm. 2016. PMID: 26773599
-
Levofloxacin loaded chitosan and poly-lactic-co-glycolic acid nano-particles against resistant bacteria: Synthesis, characterization and antibacterial activity.J Infect Public Health. 2024 May;17(5):906-917. doi: 10.1016/j.jiph.2024.03.023. Epub 2024 Mar 22. J Infect Public Health. 2024. PMID: 38569270
-
Modified nanoprecipitation method to fabricate DNA-loaded PLGA nanoparticles.Drug Dev Ind Pharm. 2009 Nov;35(11):1375-83. doi: 10.3109/03639040902939221. Drug Dev Ind Pharm. 2009. PMID: 19832638
-
Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation.Curr Drug Deliv. 2010 Jan;7(1):51-64. doi: 10.2174/156720110790396517. Curr Drug Deliv. 2010. PMID: 20044908 Review.
-
Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery.Curr Drug Deliv. 2004 Oct;1(4):321-33. doi: 10.2174/1567201043334605. Curr Drug Deliv. 2004. PMID: 16305394 Review.
Cited by
-
Preparation and Antibacterial Activity Evaluation of 18-β-glycyrrhetinic Acid Loaded PLGA Nanoparticles.Iran J Pharm Res. 2015 Spring;14(2):373-83. Iran J Pharm Res. 2015. PMID: 25901144 Free PMC article.
-
A novel approach for antibody nanocarriers development through hydrophobic ion-pairing complexation.J Microencapsul. 2014;31(6):542-50. doi: 10.3109/02652048.2014.885606. Epub 2014 Apr 3. J Microencapsul. 2014. PMID: 24697179 Free PMC article.
-
Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics.Front Chem. 2021 Feb 23;9:629635. doi: 10.3389/fchem.2021.629635. eCollection 2021. Front Chem. 2021. PMID: 33708759 Free PMC article. Review.
-
Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines.Nanomedicine. 2015 Apr;11(3):715-29. doi: 10.1016/j.nano.2014.12.013. Epub 2015 Jan 31. Nanomedicine. 2015. PMID: 25652894 Free PMC article. Review.
-
Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs' Dosage Ratio Effect.Molecules. 2019 Mar 15;24(6):1035. doi: 10.3390/molecules24061035. Molecules. 2019. PMID: 30875934 Free PMC article. Review.
References
-
- Kim BK, Hwang SJ, Park JB, Park HJ. Characteristics of felodipine-located poly (epsilon-caprolactone) microspheres. J Microencapsul. 2005;22(2):193–203. - PubMed
-
- Owusu-Ababio G, Rogers JA. Formulation and release kinetics of cephalexin monohydrate from biodegradable polymeric microspheres. J Microencapsul. 1996;13(2):195–205. - PubMed
-
- Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–289. - PubMed
-
- Dinarvand R, Alimorad MM, Amanlou M, Akbari H. Preparation, characterization and in vitro drug release properties of polytrimethylene carbonate/polyadipic anhydride blend microspheres. J Appl Polym Sci. 2006;101(4):2377–2383.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

