Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression
- PMID: 22275864
- PMCID: PMC3261917
- DOI: 10.1371/journal.ppat.1002417
Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression
Abstract
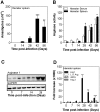
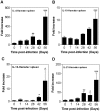
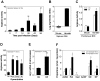
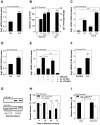
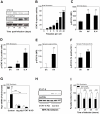
The clinicopathological features of the hamster model of visceral leishmaniasis (VL) closely mimic active human disease. Studies in humans and hamsters indicate that the inability to control parasite replication in VL could be related to ineffective classical macrophage activation. Therefore, we hypothesized that the pathogenesis of VL might be driven by a program of alternative macrophage activation. Indeed, the infected hamster spleen showed low NOS2 but high arg1 enzyme activity and protein and mRNA expression (p<0.001) and increased polyamine synthesis (p<0.05). Increased arginase activity was also evident in macrophages isolated from the spleens of infected hamsters (p<0.05), and arg1 expression was induced by L. donovani in primary hamster peritoneal macrophages (p<0.001) and fibroblasts (p<0.01), and in a hamster fibroblast cell line (p<0.05), without synthesis of endogenous IL-4 or IL-13 or exposure to exogenous cytokines. miRNAi-mediated selective knockdown of hamster arginase 1 (arg1) in BHK cells led to increased generation of nitric oxide and reduced parasite burden (p<0.005). Since many of the genes involved in alternative macrophage activation are regulated by Signal Transducer and Activator of Transcription-6 (STAT6), and because the parasite-induced expression of arg1 occurred in the absence of exogenous IL-4, we considered the possibility that L. donovani was directly activating STAT6. Indeed, exposure of hamster fibroblasts or macrophages to L. donovani resulted in dose-dependent STAT6 activation, even without the addition of exogenous cytokines. Knockdown of hamster STAT6 in BHK cells with miRNAi resulted in reduced arg1 mRNA expression and enhanced control of parasite replication (p<0.0001). Collectively these data indicate that L. donovani infection induces macrophage STAT6 activation and STAT6-dependent arg1 expression, which do not require but are amplified by type 2 cytokines, and which contribute to impaired control of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

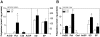
References
-
- Barbosa Junior AA, Andrade ZA, Reed SG. The pathology of experimental visceral leishmaniasis in resistant and susceptible lines of inbred mice. Braz J Med Biol Res. 1987;20:63–72. - PubMed
-
- Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, et al. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. - PubMed
-
- Stern JJ, Oca MJ, Rubin BY, Anderson SL, Murray HW. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988;140:3971–3977. - PubMed
-
- Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

