Adaptation and preadaptation of Salmonella enterica to Bile
- PMID: 22275872
- PMCID: PMC3261920
- DOI: 10.1371/journal.pgen.1002459
Adaptation and preadaptation of Salmonella enterica to Bile
Abstract
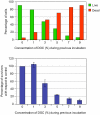
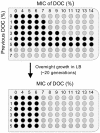
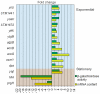
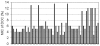
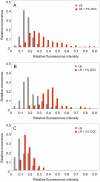
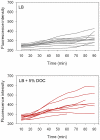
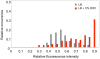
Bile possesses antibacterial activity because bile salts disrupt membranes, denature proteins, and damage DNA. This study describes mechanisms employed by the bacterium Salmonella enterica to survive bile. Sublethal concentrations of the bile salt sodium deoxycholate (DOC) adapt Salmonella to survive lethal concentrations of bile. Adaptation seems to be associated to multiple changes in gene expression, which include upregulation of the RpoS-dependent general stress response and other stress responses. The crucial role of the general stress response in adaptation to bile is supported by the observation that RpoS(-) mutants are bile-sensitive. While adaptation to bile involves a response by the bacterial population, individual cells can become bile-resistant without adaptation: plating of a non-adapted S. enterica culture on medium containing a lethal concentration of bile yields bile-resistant colonies at frequencies between 10(-6) and 10(-7) per cell and generation. Fluctuation analysis indicates that such colonies derive from bile-resistant cells present in the previous culture. A fraction of such isolates are stable, indicating that bile resistance can be acquired by mutation. Full genome sequencing of bile-resistant mutants shows that alteration of the lipopolysaccharide transport machinery is a frequent cause of mutational bile resistance. However, selection on lethal concentrations of bile also provides bile-resistant isolates that are not mutants. We propose that such isolates derive from rare cells whose physiological state permitted survival upon encountering bile. This view is supported by single cell analysis of gene expression using a microscope fluidic system: batch cultures of Salmonella contain cells that activate stress response genes in the absence of DOC. This phenomenon underscores the existence of phenotypic heterogeneity in clonal populations of bacteria and may illustrate the adaptive value of gene expression fluctuations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Adaptation of Salmonella enterica to bile: essential role of AcrAB-mediated efflux.Environ Microbiol. 2018 Apr;20(4):1405-1418. doi: 10.1111/1462-2920.14047. Epub 2018 Feb 2. Environ Microbiol. 2018. PMID: 29349886
-
Mutational and non mutational adaptation of Salmonella enterica to the gall bladder.Sci Rep. 2019 Mar 26;9(1):5203. doi: 10.1038/s41598-019-41600-8. Sci Rep. 2019. PMID: 30914708 Free PMC article.
-
Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence.J Bacteriol. 2003 Sep;185(17):5328-32. doi: 10.1128/JB.185.17.5328-5332.2003. J Bacteriol. 2003. PMID: 12923112 Free PMC article.
-
Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts.Front Med (Lausanne). 2017 Oct 3;4:163. doi: 10.3389/fmed.2017.00163. eCollection 2017. Front Med (Lausanne). 2017. PMID: 29043249 Free PMC article. Review.
-
Global regulators and environmental adaptation in Gram-negative pathogens.Clin Microbiol Infect. 2009 Jan;15 Suppl 1:47-50. doi: 10.1111/j.1469-0691.2008.02684.x. Clin Microbiol Infect. 2009. PMID: 19220355 Review.
Cited by
-
Campylobacter jejuni benefits from the bile salt deoxycholate under low-oxygen condition in a PldA dependent manner.Gut Microbes. 2023 Dec;15(2):2262592. doi: 10.1080/19490976.2023.2262592. Epub 2023 Sep 28. Gut Microbes. 2023. PMID: 37768138 Free PMC article.
-
New Adapted In Vitro Technology to Evaluate Biofilm Formation and Antibiotic Activity Using Live Imaging under Flow Conditions.Diagnostics (Basel). 2021 Sep 23;11(10):1746. doi: 10.3390/diagnostics11101746. Diagnostics (Basel). 2021. PMID: 34679444 Free PMC article.
-
Food-to-Humans Bacterial Transmission.Microbiol Spectr. 2020 Jan;8(1):10.1128/microbiolspec.mtbp-0019-2016. doi: 10.1128/microbiolspec.MTBP-0019-2016. Microbiol Spectr. 2020. PMID: 31950894 Free PMC article. Review.
-
Conflicting roles for a cell surface modification in Salmonella.Mol Microbiol. 2013 Jun;88(5):970-83. doi: 10.1111/mmi.12236. Epub 2013 May 5. Mol Microbiol. 2013. PMID: 23646936 Free PMC article.
-
Spinal Cord Injury Changes the Structure and Functional Potential of Gut Bacterial and Viral Communities.mSystems. 2021 May 11;6(3):e01356-20. doi: 10.1128/mSystems.01356-20. mSystems. 2021. PMID: 33975974 Free PMC article.
References
-
- Hofmann AF. Bile secretion and enterohepatic circulation of bile acids. In: Feldman M, Scharschmidt BF, Sleisenger WB, editors. Sleisenger and Fordtran's Gastrointestinal Disease, 6th ed. Philadelphia, PA: W. B. Saunders & Co; 1998. pp. 937–948.
-
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. - PubMed
-
- Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009;58:1533–1541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

