Insulin signaling regulates fatty acid catabolism at the level of CoA activation
- PMID: 22275878
- PMCID: PMC3261918
- DOI: 10.1371/journal.pgen.1002478
Insulin signaling regulates fatty acid catabolism at the level of CoA activation
Abstract
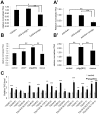
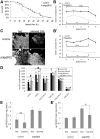
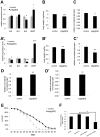
The insulin/IGF signaling pathway is a highly conserved regulator of metabolism in flies and mammals, regulating multiple physiological functions including lipid metabolism. Although insulin signaling is known to regulate the activity of a number of enzymes in metabolic pathways, a comprehensive understanding of how the insulin signaling pathway regulates metabolic pathways is still lacking. Accepted knowledge suggests the key regulated step in triglyceride (TAG) catabolism is the release of fatty acids from TAG via the action of lipases. We show here that an additional, important regulated step is the activation of fatty acids for beta-oxidation via Acyl Co-A synthetases (ACS). We identify pudgy as an ACS that is transcriptionally regulated by direct FOXO action in Drosophila. Increasing or reducing pudgy expression in vivo causes a decrease or increase in organismal TAG levels respectively, indicating that pudgy expression levels are important for proper lipid homeostasis. We show that multiple ACSs are also transcriptionally regulated by insulin signaling in mammalian cells. In sum, we identify fatty acid activation onto CoA as an important, regulated step in triglyceride catabolism, and we identify a mechanistic link through which insulin regulates lipid homeostasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation.Biochim Biophys Acta. 2007 Mar;1771(3):286-98. doi: 10.1016/j.bbalip.2006.05.003. Epub 2006 May 16. Biochim Biophys Acta. 2007. PMID: 16798075 Review.
-
Functional analysis of long-chain acyl-CoA synthetase 1 in 3T3-L1 adipocytes.J Biol Chem. 2009 Jul 3;284(27):18347-56. doi: 10.1074/jbc.M109.017244. Epub 2009 May 8. J Biol Chem. 2009. PMID: 19429676 Free PMC article.
-
FOXO regulates organ-specific phenotypic plasticity in Drosophila.PLoS Genet. 2011 Nov;7(11):e1002373. doi: 10.1371/journal.pgen.1002373. Epub 2011 Nov 10. PLoS Genet. 2011. PMID: 22102829 Free PMC article.
-
Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking.Circulation. 2019 Jun 11;139(24):2765-2777. doi: 10.1161/CIRCULATIONAHA.119.039610. Epub 2019 Mar 26. Circulation. 2019. PMID: 30909726 Free PMC article.
-
Understanding Forkhead box class O function: lessons from Drosophila melanogaster.Antioxid Redox Signal. 2011 Feb 15;14(4):635-47. doi: 10.1089/ars.2010.3407. Epub 2010 Oct 20. Antioxid Redox Signal. 2011. PMID: 20618068 Review.
Cited by
-
Triacylglycerol Metabolism in Drosophila melanogaster.Genetics. 2018 Dec;210(4):1163-1184. doi: 10.1534/genetics.118.301583. Genetics. 2018. PMID: 30523167 Free PMC article. Review.
-
Loss of function in the Drosophila clock gene period results in altered intermediary lipid metabolism and increased susceptibility to starvation.Cell Mol Life Sci. 2020 Dec;77(23):4939-4956. doi: 10.1007/s00018-019-03441-6. Epub 2020 Jan 20. Cell Mol Life Sci. 2020. PMID: 31960114 Free PMC article.
-
Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes.Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17959-64. doi: 10.1073/pnas.1409241111. Epub 2014 Dec 3. Proc Natl Acad Sci U S A. 2014. PMID: 25472843 Free PMC article.
-
Genome assembly of Genji firefly (Nipponoluciola cruciata) reveals novel luciferase-like luminescent proteins without peroxisome targeting signal.DNA Res. 2024 Apr 1;31(2):dsae006. doi: 10.1093/dnares/dsae006. DNA Res. 2024. PMID: 38494174 Free PMC article.
-
L-Carnitine in Drosophila: A Review.Antioxidants (Basel). 2020 Dec 21;9(12):1310. doi: 10.3390/antiox9121310. Antioxidants (Basel). 2020. PMID: 33371457 Free PMC article. Review.
References
-
- Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol. 2009;41:1006–1010. - PubMed
-
- Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835. - PubMed
-
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. - PubMed
-
- Newsholme EA, Dimitriadis G. Integration of biochemical and physiologic effects of insulin on glucose metabolism. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S122–134. - PubMed
-
- Pascale A, Pais R, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis. 2010;19:415–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

