Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress
- PMID: 22275901
- PMCID: PMC3257848
- DOI: 10.3389/fphar.2011.00096
Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress
Abstract
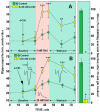
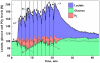
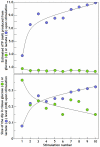
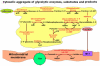
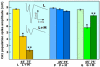
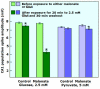
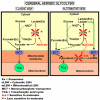
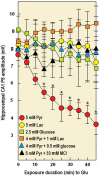
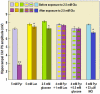
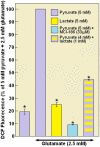
Ever since it was shown for the first time that lactate can support neuronal function in vitro as a sole oxidative energy substrate, investigators in the field of neuroenergetics have been debating the role, if any, of this glycolytic product in cerebral energy metabolism. Our experiments employed the rat hippocampal slice preparation with electrophysiological and biochemical methodologies. The data generated by these experiments (a) support the hypothesis that lactate, not pyruvate, is the end-product of cerebral aerobic glycolysis; (b) indicate that lactate plays a major and crucial role in affording neural tissue to respond adequately to glutamate excitation and to recover unscathed post-excitation; (c) suggest that neural tissue activation is accompanied by aerobic lactate and NADH production, the latter being produced when the former is converted to pyruvate by mitochondrial lactate dehydrogenase (mLDH); (d) imply that NADH can be utilized as an endogenous scavenger of reactive oxygen species (ROS) to provide neuroprotection against ROS-induced neuronal damage.
Keywords: LDH; NADH; glycolysis; hippocampal slice; lactate; neuronal function; neuroprotection; reactive oxygen species.
Figures
References
-
- Atlante A., de Bari L., Bobba A., Marra E., Passarella S. (2007). Transport and metabolism of l-lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim. Biophys. Acta 1767, 1285–1299 10.1016/j.bbabio.2007.08.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources

